Chemistry has always played a pivotal role in driving innovation across industries. Decades ago, 2,3,6-Trimethylphenol caught the attention of scientists due to its unique substitution pattern on the benzene ring. Researchers in the mid-20th century began to recognize that subtle changes to the basic phenol structure could open doors to a range of downstream products. Back then, the search for new additives in lubricants, solvents, and pharmaceutical intermediates led to a flurry of phenol methylation studies. Among them, the trialkyl variants, like 2,3,6-Trimethylphenol, stood out because of their distinct reactivity and stability compared to simple phenols. Advances in catalysis and organic synthesis only strengthened the significance of this compound, moving it from a laboratory curiosity to a staple in modern organic synthesis and specialty chemical production.
2,3,6-Trimethylphenol occupies a unique niche in the specialty chemical landscape. It’s more than just a molecule; it serves as a bridge between basic organic chemistry and advanced material science. In industrial settings, 2,3,6-Trimethylphenol gets sourced for its role in the manufacture of antioxidants, agrochemicals, and as an intermediate for pharmaceutical compounds. Its unique structure allows chemists to customize reactivity, making it a sought-after raw material for various synthesis routes. Many end users depend on the reliability and high purity that large-scale manufacturers bring to the table, making quality assurance an ever-present concern.
The molecular formula for 2,3,6-Trimethylphenol reads as C9H12O, with a molecular weight around 136.19 g/mol. This aromatic compound presents as a crystalline or oily solid at room temperature, often showing a slight yellow tint. The melting point hovers close to 40°C, while its boiling point surpasses 220°C. Solubility trends lean toward moderate in organic solvents like ethanol and ether but limited in water. Three methyl groups ringed around the phenol core amplify its hydrophobicity and tweak its electron density—a factor that affects both solubility and chemical reactivity. The dense, sweet phenolic odor is instantly recognizable in a lab environment.
Professionals working with 2,3,6-Trimethylphenol track batch purity through high-performance liquid chromatography and gas chromatography, with commercial grades often claiming upwards of 98% purity. Color standards, trace metals, and water content demand rigorous controls, as these parameters directly influence downstream reactions. Packaging involves inert, sealed containers—often amber glass or high-density polyethylene—to fend off UV light and atmospheric moisture. Clear labeling includes lot number, production date, country of origin, and full chemical naming, which goes beyond compliance and lets buyers match material consistency across projects—something any experienced chemist knows saves headaches later.
Traditionally, methylation of phenol or dimethylphenol precursors gives rise to 2,3,6-Trimethylphenol. In practice, manufacturers employ alkylation reactions, where phenol or o-cresol gets treated with methylating agents like methyl iodide, dimethyl sulfate, or methanol in the presence of acid or basic catalysts. Solid acid catalysts, such as zeolites, have boosted selectivity and yield, letting chemists sidestep the tedium of laborious purification. Multi-step syntheses also appear in patents, where careful temperature control and phase separation are essential to maximizing output while minimizing tars and byproducts. For companies emphasizing green chemistry, continuous flow reactors and less toxic reagents represent the next leap in both safety and efficiency.
With its electron-rich aromatic ring, 2,3,6-Trimethylphenol welcomes a range of chemical attacks. Ethers form readily under Williamson synthesis conditions, while mild oxidizing agents target the methyl groups, opening the door to carboxylic acid derivatives. Sulfonation becomes less straightforward due to the steric bulk of three methyl groups, demonstrating how subtle ring substitutions impact functionalization. Skilled chemists use this molecule as a launchpad, often introducing further substituents at the 4- or 5-position to create advanced materials with finely tuned properties. These downstream products feed into dyes, pharmaceuticals, and UV stabilizers.
2,3,6-Trimethylphenol comes packed with a list of aliases, such as 2,3,6-trimethylhydroxybenzene, 2-hydroxy-1,3,5-trimethylbenzene, and its shorter moniker, mesitol. Different industries or suppliers may choose their preferred names, but the chemical remains the same. Some catalogues list it by its CAS number, 2416-94-6, ensuring no confusion during procurement. These alternate titles have tripped up more than one procurement officer, making consistency in communication between purchasing and laboratory crucial.
In the real world, nobody wants an incident in the workplace, especially with organic chemicals. 2,3,6-Trimethylphenol deserves respect, with standard hazard pictograms calling out its potential skin and eye irritant properties. Material safety data sheets point out the need for well-ventilated areas, chemical splash goggles, gloves, and lab coats. Spillage protocols stress immediate containment and removal, avoiding contamination of drains. Regulatory guidelines drive disposal through certified chemical waste routes. In labs I’ve worked, safety officers never compromise on regular training and clear signage—it keeps everyone sharp and accidents rare.
Demand for 2,3,6-Trimethylphenol mainly streams in from the specialty chemical, pharmaceutical, and agricultural sectors. This compound supports production of antioxidants for plastics and rubbers, protecting materials from breakdown under stress or environmental exposure. Agrochemical formulators draw on its phenolic core when engineering new fungicides and herbicides. In pharmaceuticals, it acts as a precursor for pain relievers, antiseptics, or as a synthetic stepping-stone to larger, bioactive molecules. Laboratory research on organic synthesis often references 2,3,6-Trimethylphenol not just for its direct reactivity, but as a model substrate in mechanistic studies.
Universities and corporate R&D teams invest in understanding both the limitations and advantages of 2,3,6-Trimethylphenol. Every successful new route or catalyst broadens the substrate’s application map, keeping it in the running as a go-to building block. Scientists search for greener, faster, and more selective synthesis, cutting out hazardous reagents, waste, and energy use. Analytical teams keep refining detection and quantification methods for impurities, which in turn translate to improvements in downstream process reliability. I’ve seen firsthand how even modest adjustments—better purification steps, for instance—yield significant improvements across production chains.
Toxicologists haven’t glossed over 2,3,6-Trimethylphenol’s profile. Laboratory animals exposed to excess doses have shown mild, reversible symptoms—mainly skin or mucous membrane irritation. Long-term bioaccumulation looks unlikely thanks to its metabolic breakdown in mammals, but no prudent operator treats any phenol derivative lightly. Regulatory agencies push for ongoing assessments, especially as usage grows in consumer-facing products. In my experience, transparent toxicity reporting and continuous monitoring build trust with both the public and regulators. Risk management depends on up-to-date data and clear communication across supply chains.
Looking ahead, the trajectory for 2,3,6-Trimethylphenol seems tied closely to trends in green chemistry and smarter manufacturing. As industries push for less waste and lower energy inputs, the drive for efficient, recyclable catalysts and renewable feedstocks puts pressure on traditional producers to innovate. Demand for high-performance plastics, pharmaceuticals with custom-tailored properties, and next-generation agroformulations creates an ever-widening set of targets for material scientists. Continuous flow synthesis, machine learning-guided process development, and lifecycle toxicity assessments will likely shape the evolution of this chemical over the next decade. Striking the balance between sustainability and performance feels more urgent each year, not just for regulatory compliance, but for a company’s social responsibility reputation and long-term economic survival.
Some chemicals stay tucked away in labs, but 2,3,6-Trimethylphenol turns up in places most people wouldn’t expect. I’ve seen it shape processes in pharmaceuticals, dyes, and a corner of the plastics world. Its unique molecular structure—those three methyl groups attached to the phenol ring—gives it properties others can’t easily mimic.
Vitamin E appears everywhere from fortified cereals to skin creams, and its demand never seems to shrink. The backbone of industrial vitamin E production relies on synthetic routes using 2,3,6-Trimethylphenol. Manufacturers prize it as a starting material for the synthesis of trimethylhydroquinone, a key intermediate in making vitamin E. Without this compound, the process chokes up, and the cost of vitamin E jumps. Supplying a global consumer market counts on stable sources of this chemical.
Step into a textile dye shop or a car paint workshop and you’re surrounded by sharp colors—orange, red, and purple. These shades begin life in chemical vats where aromatic compounds like 2,3,6-Trimethylphenol serve as building blocks. It reacts predictably, letting chemists tailor-make unique dyes. Color consistency matters in fashion, and this compound helps brands keep their shades the same across batches and seasons.
On the plastics line, 2,3,6-Trimethylphenol pops up in specialty polymers. Its structure adds stability, making plastic tough and resistant to weathering and heat. Car manufacturers and electronics designers benefit most; they can offer goods with longer lifespans and fewer recalls. Polycarbonate resins, in particular, have seen advances thanks to this compound—stronger screens and safer, shatter-resistant components are a direct result.
Medicines change lives, and minor tweaks in chemical structure can mean the difference between a blockbuster drug and a failed experiment. Drug innovators use 2,3,6-Trimethylphenol as a starting point or intermediate, especially for compounds that demand multiple methyl groups for their activity or stability. Its predictable behavior during synthesis speeds up research and keeps costs lower.
Even practical chemicals bring problems. A big industry challenge remains waste generated during production and downstream use. Phenolic compounds, if released untreated, stress water supplies and local ecosystems. Companies that produce or use 2,3,6-Trimethylphenol have started investing in greener processes. Some switch to closed-loop systems, which recycle and reuse solvents. Others explore catalysts that generate fewer by-products, making factories safer for workers and neighbors. In my work with chemical safety audits, robust community engagement around emissions reporting always helped smooth tensions and encouraged new solutions.
Demand for vitamins and specialty plastics keeps rising, so the profile of 2,3,6-Trimethylphenol will only climb higher. Newer chemistries promise lower energy costs and smarter waste management. The best future for this compound connects innovative chemistry with a strong sense of responsibility, both toward end users and the planet.
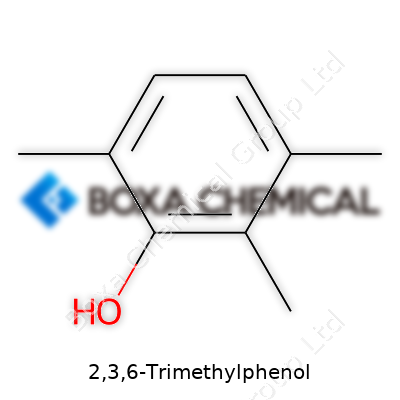
2,3,6-Trimethylphenol comes with a chemical formula of C9H12O. You’ve got a benzene ring at its core, three methyl groups attached, and a hydroxyl group making its presence felt. These adjustments make the molecule special—both in how it behaves and what you can do with it.
The molecular weight stands at 136.19 grams per mole. Jump into the calculations—each carbon brings along 12.01, hydrogen gives 1.008, and oxygen adds 16. Put them together and you won’t miss the mark by much. People handle this compound daily in labs or manufacturing plants without thinking twice about its exact weight, but accuracy matters. High-precision research or industrial production never skimps on the math.
This compound gets some attention in specialty chemical circles, mainly due to its role in synthesizing antioxidants and dyes. Labs turn to 2,3,6-Trimethylphenol because those methyl groups shift chemical reactivity. You don’t see this molecule on grocery shelves, but its behind-the-scenes work helps shape products people use all the time—think stabilizers in plastics or additives that boost product shelf life.
From personal experience, small tweaks in formula or molecular weight catch up quickly. I once watched a team mix up 2,3,6-Trimethylphenol with another variant, sending a research project into a tailspin. They learned the hard way—chemical identity always matters, and accuracy in data like molecular weight can make or break entire batches.
Product safety goes hand-in-hand with clear information. All the data sheets and safety protocols list compound details down to the last decimal. Inconsistent or incorrect information about something basic—like molecular weight—spells disaster. The right numbers mean chemical engineers figure out exact concentrations, researchers hit the outcomes they planned for, and plant operators don’t face downtime from a miscalculation.
Problems pop up when companies lean too hard on outdated or unchecked sources. PubChem, ChemSpider, and printed lab manuals don’t line up all the time. False data at the foundation leads to all sorts of unexpected behavior in experiments and industrial processes, and costs stack up fast. Over the years, I've seen routine audits flag bottles with dubious formulae—out they go, no second chances.
You only get strong results through ongoing verification. Open databases have trimmed down the mystery, letting labs cross-check formula and weight at the click of a button. Responsible teams keep their own reference libraries up to date and share corrections immediately. That’s what helps build a reputation for reliability and quality.
Looking ahead, the smartest path for anyone handling organic compounds starts with a habit of verification. Pull trusted references, make every calculation count, and share updates fast. In the end, precision at this stage opens doors to safer, more creative, and more profitable lab or industrial outcomes.
2,3,6-Trimethylphenol sounds like something out of a lab manual. This chemical pops up in making dyes, antioxidants, and other additives. Factories bring it together in bulk, and workers dealing with it know about its sharp, medicinal smell. Some folks have seen it listed on material safety sheets, but for the general public, it stays tucked in the background.
When a chemical like this ends up under the microscope, it’s for good reason. Exposure worries center on inhalation and skin contact during manufacturing work. Breathing in its vapors or getting it on bare skin isn’t pleasant; irritation and redness show up quickly. Studies running tests on rodents point to toxic effects from high doses, like damage to liver and kidneys. Scientists haven't proven any links to cancer in people, but a lot remains unknown for long-term, low-level exposure.
I’ve worked around industrial chemicals, and the routine for personal safety always starts with gloves, goggles, and good ventilation. The Material Safety Data Sheets guide us, spelling out where risk sits. For chemicals like 2,3,6-Trimethylphenol, the rules are there for a reason, even if most folks never see the production floor. I remember once a coworker forgot his gloves and ended up with a nasty rash for days. That drove home why safety steps matter for anyone close to these raw ingredients.
Dumping industrial chemicals into waterways or soil never made sense to me. 2,3,6-Trimethylphenol resists breaking down in water and soil, so plants and animals can take it up, especially in areas near factories. Fish exposed to phenols have shown altered behavior and growth problems, which can mess up whole aquatic food webs. I’ve read reports of bioaccumulation—where small fish take in the chemical, bigger fish eat those, and the stuff climbs up the chain, ending up on our dinner plates.
One EPA study found that, while the substance doesn’t stick around forever, it still puts a strain on treatment plants. Runoff from manufacturing needs careful monitoring, and plenty of watchdog groups push for stricter waste management rules. The goal: keep chemicals like this out of tap water and off farmland.
Companies producing or using 2,3,6-Trimethylphenol must follow clear guidelines. OSHA, the EPA, and European agencies set exposure limits and basic protections in the workplace. Yet, accidental spills or leaks do happen. The public depends on emergency responders and local authorities to react fast, containing and cleaning the mess before it spreads.
Stronger upstream solutions make a difference. Research teams keep looking for greener alternatives, or new ways to break down stubborn chemicals before they get out into rivers or air. In places where factories upgraded their processes, levels of phenols in wastewater dropped sharply over time. Turning lessons into action brought real improvements for local ecosystems and kept communities safer.
For individual safety and the environment, information brings power. If you live near an industrial site or work around these substances, learning about what you’re exposed to and how to stay protected makes a real difference. The more we know and the tighter the regulations, the less we have to worry about hidden threats in everyday life.
Anyone working near chemicals ends up learning some tough lessons about safety. 2,3,6-Trimethylphenol doesn’t pop up in the mainstream news much, but it’s common in chemical manufacturing and research. Looking at its physical properties and health impacts, it’s clear this compound should never get treated as ordinary. Skin rashes, eye irritation, and breathing trouble can arise from just a spill or a mistake during handling. It pays to handle things right.
A quick look at safety data helps. This chemical sits in solid form at room temperature, but that’s where the good news ends. Breathing in dust or letting it touch the skin can lead to major discomfort. Studies on similar methylphenols show even low exposures set off allergic reactions or toxicity. Workers managing fine organic powders, myself included, all know the kind of sneezing fits and skin itching a single error causes.
Given these clear warnings, workplaces easily grow lax over time, especially if nothing bad has happened yet. The best labs and plants push against this kind of complacency. They invest time in regular retraining and don’t brush off small mistakes.
Nobody wants a fire or a health scare. 2,3,6-Trimethylphenol catches fire fairly easily, so keeping it away from open flames, sparks, or hot plates stands as a basic rule. Closed, labeled containers make a huge difference. Once I watched a rushed coworker pour directly from a poorly marked jar, and we spent half a shift searching for the right emergency guidance. Strong, well-marked containers with tight lids cut out so much risk.
A cool, dry, and well-ventilated spot in the chemical storage area works best. Humid or hot environments can mess with stability or lead to fumes filling the space. No one wants to deal with contaminated air or, even worse, stacking incompatible chemicals together — that’s how you wind up with dangerous reactions.
Gloves, goggles, and lab coats may sound overcautious, but skipping them means gambling with your own health. Even the most qualified researchers have turned back from their benches after feeling sudden eye stinging or skin irritation. From my own time in small production labs, everyone respected the dress code once they saw what even a splash could do.
Spills should get treated like an emergency, not as routine clean-up. Clear procedures help keep everyone calm, but fast response matters most. Having spill kits ready and accessible in storage and work spaces means a hiccup doesn’t become a crisis. Workers need to know exactly what steps to take, not fumble through binders or ask a manager.
Companies with great safety records make it easy for workers to stick to the rules. Adequate ventilation, good training, and regular audits play a real role. Supervisors who enforce risk controls and take feedback build an environment where people trust each other with their health and safety. My best jobs involved teams who talked openly about minor incidents so everyone learned before small mistakes grew big.
Safe storage and handling turn into habits through practice. Shared knowledge and respect for the compound’s power keep chemical workers safe, reduce business risks, and protect the environment. Each person’s experience builds on the last, making the entire workspace more secure.
At the core of chemical manufacturing, purity isn’t a suggestion. For 2,3,6-Trimethylphenol, holding the right purity percentage goes well beyond checkbox compliance; it shapes everything from downstream reaction performance to waste management on the factory floor. Producers often target a purity between 98% and 99% by weight for industrial-grade batches. Anything less risks unwanted side products, higher operational costs, and the sort of headaches nobody in an industrial plant wants to deal with.
In my own years working with procurement teams and plant technicians, nobody forgets how contaminants—think isomeric impurities or sticky organic leftovers—can mess with the catalyst or throw reaction yields off target. An extra two percent of mystery compound doesn’t sound like much, but the build-up builds real problems, especially in high-throughput pipelines where margins are as tight as deadlines.
Getting a pure cut of 2,3,6-trimethylphenol isn’t just a matter of pride. Routinely, labs report on “relative area percentage” from gas chromatographs. For industrial grade, that accepted range gives a little play for tars, heavy aromatics, or close-isomer relatives like 2,3,5-trimethylphenol. These can sneak in during distillation or sloppy handling of solvents. Producers stamp out anything above the allowed 1% to 2% junk content — too many surprises have taught them not to gamble with product performance or the safety of downstream processes.
Trace metals and water bring their own baggage. Market standards set water content below 0.2% and keep metal ions under strict limits, since these can poison catalysts or trigger off odors that even the casual observer can spot in specialty polymers or resin manufacturing. I’ve stood next to tanks where an overlooked fraction of copper or iron trashed an entire batch’s color. Nobody wants that repeat.
Companies who care about credibility let third-party laboratories run infrared and chromatographic analyses on every production lot. Some buyers even request detailed impurity profiles—not just a broad purity number—since these specifics clue them in on whether the batch suits their intended use. It’s become standard for reputable suppliers to provide certificates documenting purity, maximum impurity levels, and solvent residue analysis.
One simple lesson: don’t trust just the label or the sales pitch. Proper traceability and random re-testing aren’t just bureaucratic rituals. They’ve saved more than one job from recall or disaster, especially in markets where regulatory inspection comes without warning. Knowing your supplier’s technical approach—batch distillation versus continuous, for example—gives even modest buyers an edge in negotiating repeatable, high-quality loads.
Every production manager I’ve met wants a world where chemical purity is a given, not a guess. In reality, mistakes happen if testing equipment is outdated or if operators cut corners. Investing in stronger analytical labs or automating sampling with real-time sensors can weed out bad batches before they reach the warehouse.
Industry groups, academic partners, and frontline operators have made it clear that clear guidelines work, but they’re not magic. Sharing case studies, keeping process data transparent, and rewarding plants that consistently meet or beat the 98%-99% bar push the whole sector forward. In the end, sticking to rigorous purity standards for 2,3,6-trimethylphenol isn’t about endless red tape; it’s about making sure every player in the supply chain can count on what’s inside the drum.
| Names | |
| Preferred IUPAC name | 2,3,6-Trimethylphenol |
| Other names |
2,3,6-Trimethylphenol 2,3,6-Trimethyl-1-hydroxybenzene Mesitol Phenol, 2,3,6-trimethyl- |
| Pronunciation | /tuː, θriː, sɪks-traɪˈmɛθɪlˌfiːnɒl/ |
| Identifiers | |
| CAS Number | 527-60-6 |
| Beilstein Reference | 1209221 |
| ChEBI | CHEBI:34262 |
| ChEMBL | CHEMBL16275 |
| ChemSpider | 16252 |
| DrugBank | DB04218 |
| ECHA InfoCard | EC 202-609-9 |
| EC Number | 1.14.13.25 |
| Gmelin Reference | 1640 |
| KEGG | C01746 |
| MeSH | D015555 |
| PubChem CID | 6974 |
| RTECS number | GO8575000 |
| UNII | 7C896481F0 |
| UN number | UN2326 |
| CompTox Dashboard (EPA) | DTXSID7020153 |
| Properties | |
| Chemical formula | C9H12O |
| Molar mass | 150.22 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | phenolic |
| Density | 0.994 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 1.9 |
| Vapor pressure | 0.09 mmHg (25°C) |
| Acidity (pKa) | 10.65 |
| Basicity (pKb) | 8.57 |
| Magnetic susceptibility (χ) | -62.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.535 |
| Viscosity | 1.22 mPa·s (at 25 °C) |
| Dipole moment | 1.69 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 156.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -204.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4325 kJ/mol |
| Pharmacology | |
| ATC code | N07XX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P273, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2,3,0,0 |
| Flash point | Flash point: 113 °C |
| Autoignition temperature | 515 °C |
| Explosive limits | 1.1–6.6% (in air) |
| Lethal dose or concentration | LD50 oral rat 2960 mg/kg |
| LD50 (median dose) | Rat intravenous LD50 370 mg/kg |
| NIOSH | ZE2450000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,3,6-Trimethylphenol: Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) for 2,3,6-Trimethylphenol is "2 ppm (10 mg/m³)". |
| Related compounds | |
| Related compounds |
Phenol 2,6-Xylenol 2,3,5-Trimethylphenol 2,4,6-Trimethylphenol |