Chemists chasing unique aromatic compounds in the early 20th century started to focus on methyl-substituted phenols, driven by both curiosity and the growing needs of synthetic chemistry. Through the decades, discovery followed industry trends—synthetic dyes, resins, and antioxidants gained ground, spreading curiosity about the methylphenol family. Among them, 2,3,5-Trimethylphenol drew attention as both a precursor and a functional molecule, edging into use as methods for selective methylation improved. The paths paved by tar distillation and coal-based methods gradually gave way to petroleum-derived chemistry. As technology advanced, the market saw increased purity achieved not only for research but for critical industrial applications, reflecting both persistence and the incremental nature of chemical manufacturing evolution.
Often recognized for its role in specialty synthesis, 2,3,5-Trimethylphenol belongs to a branch of methylphenols valued for their tuned reactivity. Chemical manufacturers supply it for both pilot and production-scale applications, ranging from resin synthesis to intermediates for plant protection products. In labs and plants, the substance answers the call for a reliable methyl donor and a bulkier phenol ring that provides steric hindrance useful in tailoring polymers or additives.
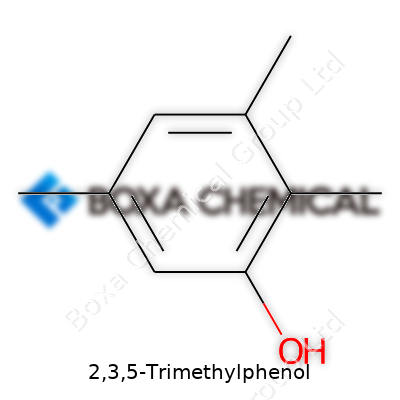
Solid at room temperature, 2,3,5-Trimethylphenol appears as a white to off-white crystalline solid. The faint, characteristic phenolic smell stays noticeable, a reminder of its benzene heritage. With a melting point hovering near 69°C and a boiling point reaching up toward 245°C, it maintains decent thermal stability in most synthetic conditions. Low water solubility sets it apart from simpler phenols, while solubility in alcohols and solvents like ether broadens its compatibility. Its chemical backbone, the three methyl groups anchoring at the second, third, and fifth positions of the ring, shapes both its behavior and handling requirements. This arrangement shapes electronic effects, influencing both reactivity in electrophilic substitutions and hydrogen bonding in formulation chemistry.
Supply chains demand clarity, so manufacturers stick to purity benchmarks—often exceeding 98% for the primary product, confirmed by gas chromatography. Customers look for a clearly marked CAS number—697-82-5—batch identifiers, and safety status on each drum or bottle. Spec sheets track melting range, moisture levels, color, and trace impurities like residual toluene or phenol. Proper labeling stays non-negotiable, especially as the transport of semi-volatile organics falls under regional chemical transport codes and hazard communication standards.
Synthetic routes rely on methylation of phenol or lower methylphenols, leveraging Friedel–Crafts or alkylation strategies. Some industrial lines start with m-cresol, alkylating selectively with methyl chloride using aluminum chloride as catalyst. Others tackle more controlled multi-step approaches, separating and purifying through distillation or crystallization. Continuous improvements in catalyst recycling and separation simplify the process, and greener chemistry has started to influence the selection of solvents and minimizing reliance on traditional Lewis acids when possible.
What really sets 2,3,5-Trimethylphenol apart is the way those methyl groups protect and activate different positions on the benzene ring. Nitration targets open spots, though careful reaction conditions are needed to dodge overreaction. Sulfonation, alkylation, and halogenation each unlock new intermediates for further chemistry. In my experience, working with methylated phenols offers a unique twist compared to simple phenol—reduced reactivity at certain sites can make targeted synthesis more straightforward, but can also frustrate chemists aiming for universal routes. The electron-donating character of each methyl group throws the push-pull of aromatic substitution into sharper relief, which synthetic chemists exploit to select for certain products over others.
Buyers and researchers come across a variety of names for this compound. The term “2,3,5-trimethylphenol” remains dominant, but synonyms like “2,3,5-trimethylhydroxybenzene” or “mesitol” occasionally show up in research papers and supplier catalogs. While some language variation exists between Asia and Western sources, the standard IUPAC nomenclature keeps translation disagreements from muddying global commerce.
Anyone handling 2,3,5-Trimethylphenol must give thought to personal safety and environmental responsibility. Phenolic compounds carry risks—skin and eye irritation take priority in risk assessments. Ventilating work spaces matters, as phenolic vapors may cause discomfort or low-level toxicity over long periods. Glove and goggle use isn’t negotiable. Reputable suppliers point toward GHS hazard codes and recommend transport in sealed containers with secondary containment. Emergency procedures for spills involve immediate physical cleanup followed by proper disposal, as phenolic residues stress water treatment facilities and disrupt aquatic life. Waste management, down to container cleaning and emissions control, has shifted as awareness about phenol toxicity has grown, nudging companies to rethink how they minimize losses and route liquid waste.
End users draw value from 2,3,5-Trimethylphenol’s role in advanced polymer chemistry, antioxidants for plastics, and various agrochemical syntheses. Specialty resin makers look to it for the building of thermostable phenolic resins. Lubricant manufacturers prize it for blending non-volatile antioxidants. Some academic groups experiment with it as a test substrate for novel oxidation catalysts or as a model compound in photochemical degradation studies. Agrochemical producers make use of it as an intermediate for herbicides and fungicides, while electronics firms sometimes incorporate it in the creation of tailored polymers with high thermal stability. The diversity comes from that subtle electronic tweaking thanks to the methyl groups on the aromatic ring.
Research continues to unlock new uses for 2,3,5-Trimethylphenol. Some studies test its activity as an antioxidant in polymer films meant for packaging, exploring extension of shelf lives for sensitive goods. Lab teams work on tuning catalysts to clean up methylation methods, using less hazardous agents and reducing byproduct streams. In the pharmaceutical sector, curiosity drives research into derivatives as enzyme inhibitors or scaffolds for small molecule screening, despite limited direct bioactivity. Analytical chemists seek out faster detection and quantification techniques—improvements in GC/MS, HPLC, and rapid screening methods mean industry can track quality more confidently. Patents published in the past decade push opportunities even further, describing new resin formulations, catalytic cycles, and sensor materials that owe their edge to the properties of this trimethylated phenol.
Toxicologists pay careful attention to methylphenols, as small changes in substitution can produce sharp changes in how the body or environment reacts. Existing animal studies show moderate acute toxicity, with the aromatic ring interacting with cellular enzymes in ways similar to other phenols, although the three methyl groups often reduce skin penetration relative to phenol itself. Chronic exposure data stays limited, so responsible manufacturers stick to conservative limits recommended by regulatory agencies. Waste stream monitoring is critical, as phenolic compounds, once released, pose a problem for aquatic organisms and disrupt microbial communities at trace concentrations. Research efforts focus on biodegradation pathways and the role that substitution patterns play in both persistence and breakdown rates in soil and water. Anyone involved in large-scale production or disposal quickly learns the importance of robust environmental controls and regular review of current toxicological data.
Demand for high-performance materials continues to rise, which keeps 2,3,5-Trimethylphenol solidly in the sights of innovation managers and research chemists. As industries tighten restrictions on toxic additives and push for greener chemistry, pressure grows to refine sourcing, synthesis, and waste management around phenolic chemicals. Biocatalysis and flow chemistry promise new ways to make methylated phenols with fewer environmental impacts and improved selectivity. Sustainable feedstocks could shift the economics and environmental equation, perhaps even seeing bio-aromatic sources supplying future needs. For now, the molecule’s unique combination of tunable reactivity, physical stability, and relative ease of modification earns it a seat at the table in any conversation about the next wave of specialty chemicals. Research teams continue chasing structure-activity relationships, material scientists lean on it for reliable performance, and environmental chemists work toward making its lifecycle safer from beginning to end. In this shifting landscape, compounds like 2,3,5-Trimethylphenol show just how intertwined chemistry, policy, and market need can be—each shaping the fate of even a single aromatic ring.
2,3,5-Trimethylphenol sounds like a mouthful, but this chemical shows up in more places than most people would think. Working in a lab years ago, I faced racks of sample bottles with long names. Many chemicals didn’t seem special on the surface, but manufacturers rely on them each day. 2,3,5-Trimethylphenol makes a perfect example. Using it is not flashy or exciting for the average person, but industries can’t skip it without big consequences.
One thing that stands out about 2,3,5-Trimethylphenol is its role in creating antioxidants. In the world of plastics and rubbers, oxidation can ruin a product quickly. Toss a plastic toy out in the sun, and it grows brittle in a year. Antioxidants slow this decay and keep things sturdy and flexible. 2,3,5-Trimethylphenol anchors the process of making these additives by giving chemists a stable, reactive building block. Some synthetic antioxidants like 2,6-di-tert-butyl-4-methylphenol come directly from chemicals in this family. Research and industry reports show how demand for strong, long-lasting plastics and synthetic rubbers keeps rising, pushing up the need for trimethyl-based chemicals.
Agriculture also depends on specialty chemical makers, even if many farmers never see the raw ingredients. Many pesticides and herbicides start from a phenol base before getting tweaked for performance and safety. By having methyl groups in specific spots, 2,3,5-Trimethylphenol lets chemists fine-tune how molecules interact with weeds, fungi, or pests. That careful balance between crop safety and pest control makes every tweak to the chemical backbone count. Growing up in a rural area, I saw how one bad batch or untested formula could ruin a harvest, so the right chemical intermediates make a concrete difference.
Few people connect these specialty chemicals to healthcare, yet many painkillers, cough medicines, or local anesthetics contain structures similar to 2,3,5-Trimethylphenol. Even if the final pill looks far removed, the basic chemical recipe starts here. This piece works as a starting point for reactions that shape new drugs. The reliability of these ingredients builds confidence in each dose.
Handling chemicals like 2,3,5-Trimethylphenol requires care. Toxicity reports show that unmanaged spills or accidents can create real hazards for workers and nearby communities. Factories need strict protocols, strong training, and constant monitoring—something not every facility keeps up with. People sometimes ask if these production chains could shift toward greener methods. Some researchers are investigating synthetic pathways from renewable sources, though nothing beats the efficiency of petrochemical feedstocks so far.
Chemical manufacturing should not be driven purely by profit. Stakeholders—factory personnel, local residents, public health officials—deserve updated information and a voice in safety decisions. Investments in emission controls and waste treatment pay off in both reputation and long-term cost savings.
Most people will never see a vial of 2,3,5-Trimethylphenol. They feel its impact each time they use long-lasting plastics, safer pesticides, or reliable medicines. The conversation about these building blocks needs to keep growing, with more transparency and better balance between innovation, economic needs, and public health. Companies that take the lead on accountability and safe handling can earn the trust of both regulators and consumers.
2,3,5-Trimethylphenol brings together a simple aromatic ring with three methyl groups and a single hydroxyl group. Its chemical formula reads as C9H12O. That’s three methyl groups strapped onto a six-carbon benzene ring, with one oxygen attached as a hydroxyl group. Anyone who’s paid attention in organic chemistry will recognize the phenol core. Shift those methyl groups to the right places, and the molecule turns into something with very different properties from plain phenol.
Many overlook how a minor tweak in structure leads to huge shifts in chemical behavior. Take it from someone who’s watched minor changes drive major reactions in the lab—those three methyl groups change everything about how 2,3,5-trimethylphenol participates in reactions, interacts with other chemicals, and performs in industrial processes. Toss it into a reaction vessel, and those methyl positions affect solubility, reactivity, and even the way it smells. That’s no small thing for industries focused on making fragrances, resins, or specialty chemicals.
People often take compounds for granted unless they see their practical use or potential hazards. 2,3,5-Trimethylphenol plays a role as an intermediate in dye manufacturing, antiseptics, and resin production. It’s not just a textbook example or lab oddity. Miss a safety precaution and you’re not just risking a failed reaction – exposure brings the risk of irritation to skin, eyes, and lungs. As someone with more than one chemical spill under my belt, I’ve learned not to cut corners with protective equipment, and that’s advice worth following with trimethylphenols, too.
Regulators look closely at compounds like 2,3,5-trimethylphenol because even minor environmental exposure can accumulate. Not all chemicals break down quickly or cleanly. It's critical for manufacturing facilities to track waste streams and air emissions. Both the EPA and OSHA place limits on workplace concentration and waste disposal, reflecting a growing focus on worker and public safety. Effective ventilation, waste capture, and responsible transport keep problems from spiraling.
Toxicological data on phenol derivatives, including this one, shows the threshold limit values rest below 5 ppm for workplace exposure. That standard exists because repeated or high-concentration exposure carries real risks, not just theoretical ones. Peer-reviewed literature points to oxidative stress as a driver of toxicity—something every safety manager must keep in mind when setting policies for phenolic compounds.
Zero-discharge goals and green chemistry innovations aim to replace hazardous reagents with safer alternatives. Substituting conventional phenolic compounds with bio-based options reduces exposure risk and makes processes cleaner. I’ve watched teams save time and money by switching to less hazardous inputs, investing in better containment, and regular staff training. Those shifts bring safety and environmental benefits without sacrificing product quality.
Clear communication and data sharing build public and stakeholder trust in chemical manufacturing. Research transparency, open safety records, and third-party audits help keep everyone honest. Companies embracing this model attract partnerships, investment, and loyal customers—because people want more than empty promises; they want proof. C9H12O, with its three methyls and an OH group, is just one molecule in a vast universe, but the way we handle it speaks volumes about the state of modern chemistry.
People often don’t pay much attention to names like 2,3,5-Trimethylphenol. The word itself doesn’t roll off the tongue, yet it appears in factories, research labs, and organic chemistry labs. This chemical shows up because it’s useful in making resins and other industrial products, but its usefulness doesn’t make it safe. Whenever I hear about a substance with a phenol backbone, my first thought lands on health and environmental worries. Old tales from chemistry class ring in the back of my mind—stories where chemical accidents caused burns and lingering coughs. Phenols can do that, and 2,3,5-Trimethylphenol belongs to this club.
Let’s get to the point: 2,3,5-Trimethylphenol can be harmful. Touching it or breathing in its vapors won’t feel like dipping a hand in soapy water. Eyes sting, skin may burn, throat feels raw. Even brief exposure can turn into days of irritation. The chemical itself smells harsh, which acts as an early warning, but people get used to unpleasant smells after a while. That’s what makes it riskier. You stand near an open bottle, and after a few minutes, you barely notice the odor. That’s not because it’s safe—it’s just how the nose works.
I once chatted with an old friend who worked in an adhesives plant. She described a day spent cleaning up a minor spill of this stuff. The required safety gear, from gloves to full-face respirator, made moving around clumsy and hot, but no one dared skip a step. Colleagues learned the hard way not to ignore the rules; a few cases of red, blistering skin and watery eyes hammered that lesson home.
Trimethylphenol doesn’t wait for an invitation to get into your system. It creeps in through the skin. Inhaling fumes over several hours could bring on headaches and nausea, sometimes worse. Some scientists worry about long-term damage from repeated contact, especially to the liver and kidneys. Lab tests on animals suggest these chemicals can tinker with internal organs, and though humans handle things differently than lab rats, the warning signs look too bright to ignore.
I’ve spent hours in university labs watching how chemical waste gets cleared out. Trimethylphenol isn’t just a “pour down the drain and forget about it” kind of chemical. Pouring this stuff into the local water supply can mess with aquatic life. Even small amounts cause harm, making fish sick and disturbing ecosystems. Proper disposal isn’t just a rule, it’s a responsibility. Too many accidents happen from casual habits formed during busy shifts or on a sleepy third shift when everyone’s rushing to head home.
Awareness keeps people safe. Training workers on safe storage, using fans, wearing gloves, and eye protection all matter. Keeping emergency showers and eye-wash stations in working order actually saves pain and time in the long run. Companies must monitor air quality and check that everyone understands what they’re working with. Regulators like OSHA and EPA have set exposure limits, and it makes sense to stay far below those numbers, not skate up to the edge. If you don’t take this kind of substance seriously, it can teach a harsh, real-life lesson fast.
I’ve seen a lot of chemical storerooms—each one has its character, but all share a few universal truths. Experience tells me no corners can be cut when chemicals like 2,3,5-Trimethylphenol are involved. This compound, prized for several industrial applications, brings some real risks if storage takes a back seat. Direct contact irritates eyes and skin, while vapors make the air tough to breathe. Mishandling this chemical risks not only product loss but also employee health and environmental harm.
Storing 2,3,5-Trimethylphenol means keeping things cool and dry, always away from heat sources and open flames. This chemical reacts to heat—flash points often hover around 98°C (208°F). Any rise above room temperature encourages vaporization. Good ventilation strips away fumes, so a reliable extraction fan earns its keep here. Over the years, I’ve watched proper storage conditions prevent dangerous buildup and accidents. Research from respected bodies like the National Institute for Occupational Safety and Health (NIOSH) highlights that improper storage leads to internal pressure in sealed containers, sometimes causing ruptures or leaks.
Containers matter. Use tightly sealed, corrosion-resistant drums or bottles made from glass or high-grade plastic. Containers must carry clear hazard labels—hazard diamonds, emergency contact numbers, and batch information help keep everyone in the loop. Storage rooms get frequent checks to catch leaks early. I’ve also seen smart facilities use secondary containment trays. If spills happen, these trays help stop the spread, giving workers a chance to clean up before things worsen.
Some chemicals spark trouble when stored side by side. 2,3,5-Trimethylphenol should stay away from oxidizers, acids, or strong alkalis. One slip and the wrong combination sends out toxic fumes, fires, or even explosions. National Fire Protection Association (NFPA) guidelines stress using separate storage cabinets and marked shelves to prevent accidental mixing. In my experience, color-coded systems simplify this step and cut down mistakes under pressure.
Fancy signage and extra ventilation impress nobody if the staff lacks solid training. Every worker in a lab or warehouse with 2,3,5-Trimethylphenol needs hands-on practice—not just lectures—on spill response and personal protective equipment (PPE). Gloves, chemical splash goggles, and lab coats stay on, not off. Regular drills keep nerves steady in real emergencies. I’ve noticed that teams who practice together respond faster, making incidents less likely to spiral.
Records support best practices. Keeping updated logs answers auditors and ensures supplies get rotated before expiration. Material Safety Data Sheets (MSDS) belong close to storage areas, not buried in a drawer. This habit stands out during inspections and has protected more than one site from fines or shutdowns. Legal compliance isn’t just bureaucracy; it saves lives. Agencies like OSHA demand documentation for a reason, and ignoring this step closes doors, sometimes for good.
The safest storerooms run on respect and knowledge. Clear procedures, regular maintenance, and worker buy-in form the backbone of chemical safety. Storing 2,3,5-Trimethylphenol correctly is not optional—it protects people, profits, and the wider community. Experience, data, and vigilance win this game every time.
2,3,5-Trimethylphenol packs three methyl groups onto a phenol ring, and these subtle tweaks shape how it behaves. Those working in a chemistry lab know physical properties such as melting and boiling points guide practical decisions every day. 2,3,5-Trimethylphenol melts at about 47–49°C and boils at around 238–239°C. Numbers like these affect simple things—how it’s stored, how it moves through production lines, and what containers stand up to it without breaking down or leaking vapor.
Many folks exploring organic synthesis care about more than textbook data—handling matters. A melting point close to room temperature can give headaches if a substance appears sometimes solid, sometimes liquid on a warm day. Scoop some up at 20°C, you might get crystals; nudge the thermostat, and you’re looking at a slippery puddle. Storage calls for real attention to temperature control and container material. Ignoring it can mean spills, lost product, or surprise reactions if the phenol soaks through waxed paper or eats up a thin plastic bottle.
Boiling point matters just as much in the lab, especially for purification. Distilling a molecule at nearly 240°C asks a lot from common glassware and seals; weak points give out and pose safety hazards. Purity demands careful distillation, and that means clear knowledge of boiling thresholds so the compound stays intact and unaltered. Any slip—excess heat, old gasket, wrong type flask—can waste hours or destroy a batch.
With a high boiling point, vapor pressure remains low at room temperature. This definitely cuts down on volatility, which means lab air stays safer. Not all phenols have the same comfort—some hit you right away with powerful smells or risky fumes. 2,3,5-Trimethylphenol only becomes a breathing hazard under high heat, so simple room ventilation or a covered beaker keeps problems at bay during routine use.
Heat or an open flame will push it into the air, so fire safety deserves attention wherever large volumes get handled. Phenols have a reputation for toxicity; hands-on experience with irritated skin and harsh odors stays with anyone who spends much time around them. Gloves, eye protection, and ventilation always make sense, no matter how benign the melting point might look in the safety sheet.
An understanding of these thermal points shapes smarter manufacturing and safer storage. Labs using 2,3,5-Trimethylphenol can cut waste by tuning process temperature just above the melting point during transfer, so nothing sticks to pipes or containers. This cuts both clean-up costs and the risk of exposure.
In chemical engineering, recapturing material lost in vapor form often turns into a big savings. Condensing lines must account for a boiling mark near 239°C, or else expensive product evaporates into scrubbers or fans. Calculating these points precisely even lets teams experiment with energy input, slashing waste and protecting margins.
Published sources such as the Merck Index and the CRC Handbook list consistent melting and boiling figures for 2,3,5-Trimethylphenol—melting near 47–49°C and boiling around 238–239°C. It pays to check multiple references, since impurities shift these numbers and impact what scientists see in a working lab. Experience teaches never to treat a data sheet as gospel; double-checking with actual samples helps uncover hidden problems before they hit a full-scale run.
| Names | |
| Preferred IUPAC name | 2,3,5-Trimethylphenol |
| Other names |
2,3,5-Trimethylphenol Sym-Triamylphenol Phenol, 2,3,5-trimethyl- |
| Pronunciation | /tuː, θriː, faɪv traɪˈmɛθɪlˌfiːnɒl/ |
| Identifiers | |
| CAS Number | 527-60-6 |
| Beilstein Reference | 2073882 |
| ChEBI | CHEBI:34279 |
| ChEMBL | CHEMBL206774 |
| ChemSpider | 13709994 |
| DrugBank | DB02523 |
| ECHA InfoCard | 8489e5bd-d85c-41a4-b3ad-661efcbb1a72 |
| EC Number | 1.14.13.11 |
| Gmelin Reference | 79334 |
| KEGG | C02268 |
| MeSH | D017355 |
| PubChem CID | 6945 |
| RTECS number | RD3325000 |
| UNII | Q7M1OK24T9 |
| UN number | UN2326 |
| CompTox Dashboard (EPA) | DTXSID2020084 |
| Properties | |
| Chemical formula | C9H12O |
| Molar mass | 150.22 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | Phenolic odor |
| Density | 0.982 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.94 |
| Vapor pressure | 0.0313 mmHg (25°C) |
| Acidity (pKa) | 10.7 |
| Basicity (pKb) | 8.88 |
| Magnetic susceptibility (χ) | -60.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.525 |
| Viscosity | 3.16 cP (20°C) |
| Dipole moment | 1.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 174.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -197.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3895 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362, P501 |
| Flash point | 104 °C (219 °F; 377 K) |
| Autoignition temperature | 550 °C |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2960 mg/kg (rat, oral) |
| NIOSH | SE3150000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,3,5-Trimethylphenol: Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) for 2,3,5-Trimethylphenol is: "2,3,5-Trimethylphenol: REL = 10 mg/m³ as TWA |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Phenol 2,3,6-Trimethylphenol 2,4,6-Trimethylphenol 2,3,5,6-Tetramethylphenol 2-Methylphenol (o-cresol) 3-Methylphenol (m-cresol) 4-Methylphenol (p-cresol) |