Looking into the story of 2,3,5-Trimethylhydroquinone feels like stepping through a timeline lined with breakthroughs in synthetic organic chemistry. The compound popped up in the backdrop of the 20th-century drive to unravel more efficient routes toward complex molecules for pharmaceuticals and vitamins. German researchers charted out routes to methylated hydroquinones around the 1930s and 1940s, motivated by the race to produce Vitamin E—the antioxidant that shaped everyday food and health industries. Manufacturers in Japan and Europe pushed the envelope through the 1960s and 1970s, refining production techniques that cut costs and raised purity standards. Today, the journey isn't just a matter of chemical ingenuity; it reflects the hunger for scalable solutions in sectors hungry for specialty ingredients.
2,3,5-Trimethylhydroquinone stands out among substituted hydroquinones because it fills a critical niche in synthesizing Vitamin E (α-tocopherol). Most major suppliers cater strictly to industry, setting aside small-batch academic research. The primary use hooks into the global demand for dietary supplements and fortified foods—a market tipping into tens of billions of dollars. A kilo of this compound can shape the nutritional profile for hundreds of thousands of consumer products, showing its ripple effect extends well past lab benches.
Lift the lid on a jar of pure 2,3,5-Trimethylhydroquinone and you’ll find a white to off-white crystalline solid, typically melting around 165–168°C. Insoluble in water but dissolvable in ethanol, ether, and common chloroform, it demands careful handling because of the way hydroquinones oxidize in air, especially in the presence of light or metal ions. Structurally, the three methyl groups shielding the benzene ring change reactivity compared to basic hydroquinone, making the compound less prone to some classic aromatic substitutions but still fiercely redox-active.
The path from reactor to drum emphasizes purity and trace contaminants. Specifications usually call for assay values above 98%, minimal moisture content, and full spectra in chromatographic and spectroscopic characterizations—NMR, GC-MS, HPLC. Responsible suppliers tag containers with hazard codes: avoid dust inhalation, keep away from ignition sources, and shield the product from open air and UV. Batch numbers trace back to manufacturing times, aiding recall or troubleshooting. Labels must state not just the chemical’s primary identity, but also storage protocols, recommended shelf life, and manufacturer contact in case of accidental exposure. For international shipments, Material Safety Data Sheets travel alongside.
Building 2,3,5-Trimethylhydroquinone at scale calls for well-tuned multi-step syntheses, and most industrial players start from m-cresol. Friedel-Crafts methylation plants the desired methyl groups on the aromatic ring, after which hydroxylation reactions—often with oxidants like hydrogen peroxide in acidic media—establish the hydroquinone core. Each step carries a story of yield optimization: minimizing side products, dialing in reaction times, pulling from decades of process chemistry refinement. Laboratory chemists favor milder, cleaner versions of these transformations, often exploring green catalysts or recyclable solvents to improve footprint and keep costs tighter.
Chemists eye 2,3,5-Trimethylhydroquinone for its balance of stability and modifiability. Oxidation produces the corresponding quinone, opening doors to further addition reactions or coupling processes. Reductive alkylation, selective acylations, and even enantioselective transformations turn this molecule into everything from antioxidants to specialty polymers. Sometimes, researchers tweak the methyl positioning to probe bioactivity, shifting relationships between structure and biological function—a classic move in medicinal chemistry or toxicology screens.
The molecule circles through the literature as 2,3,5-TMHQ, 2,3,5-Trimethyl-1,4-dihydroxybenzene, or simply trimethylhydroquinone. Its role as an intermediate often earns it stickers like Vitamin E intermediate or Tocopherol precursor. Regional markets play with brand names but chemistry textbooks stick to IUPAC standards. Any product sourced in the global market can usually be tracked with CAS Number 700-13-0, a key for regulatory filings and customs paperwork.
2,3,5-Trimethylhydroquinone doesn’t threaten in the class of acute toxins but routine laboratory and line workers know not to take any chances. Unprotected exposure, especially in powder form, can irritate noses, throats, and skin. Chronic contact hints at respiratory discomfort, so chemical companies enforce good local exhaust ventilation, gloves, goggles, and dust masks. European and US safety agencies demand training on chemical hygiene and strict lockup of stocks to avoid accidental cross-contamination with reactive acids or oxidizers. For transport, drums line up in cool, dry spots, away from the sun and high-traffic plant areas. Fire risk is low if material isn’t allowed to get airborne, but companies still keep extinguishers and spill kits ready.
Few specialty chemicals play such a starring role in shaping global health trends. 2,3,5-Trimethylhydroquinone’s main claim to fame is punching up tocopherols for vitamin E production. Finished tocopherols flow into food oils, margarine, breakfast cereals, infant formulas, and a cargo-load of supplements. The same synthesis skills bend this compound toward specialty antioxidants—used in cosmetics to slow skin aging, or blended into plastics and lubricants to shield against oxidative breakdown. Its impact stretches further, feeding pharmaceutical R&D for new antioxidative treatments or metabolic pathway studies. The push from emerging markets and global dietary awareness continues fueling demand, putting a premium on reliable supply and consistent product quality.
R&D teams treat 2,3,5-Trimethylhydroquinone both as a tool and as a research subject. Process chemists pursue greener, more efficient production lines—using less energy, making fewer hazardous by-products, and squeezing more yield from every kilogram of raw material. Environmental scientists look for water purification tech with hydroquinone derivatives in the mix. Biomedical labs probe the molecule’s antioxidant power, drawing lines between structure tweaks and cell-protection in models of neurodegeneration, cardiovascular health, or skin integrity. Patent filings and journal reports keep breaking new ground, often with international collaboration crossing academia and industry. The compound doesn't only drive incremental progress; it inspires process innovation and new chemistry toolkits for handling similar aromatic systems.
Hydroquinone derivatives like 2,3,5-TMHQ rarely escape toxicity testing. Early screens in rodents showed low acute toxicity, yet modern protocols dig deeper, examining potential for chronic effects, developmental toxicity, endocrine disruption, and environmental persistence. The parent hydroquinone, which appears as an impurity, drew attention for its skin depigmentation effects and links to DNA damage in sensitive test systems. Industry standards now chase lower detection thresholds for such by-products, banking on analytical advances to protect workers and the public. Toxicologists press the need for full exposure profiling—not just for the pure compound but for its breakdown products and the batch-specific contaminants that surface in industrial scale-up. Open data exchange between regulators, manufacturers, and academic researchers shapes a more informed safety environment.
The market for vitamin E isn’t cooling down. Emerging economies care more and more about food fortification, anti-aging, and chronic disease prevention. Global firms hunt for cost-cutting routes, improving feedstock efficiency, electrical usage, and waste management in 2,3,5-Trimethylhydroquinone plants. Regulatory bodies, taking cues from fresh toxicity research, keep dialing up scrutiny on purity levels and environmental stewardship. Sustainable chemistry teams now ask if they can make this compound with renewable catalysts—or tap into biobased feedstocks to trim the industry’s carbon footprint. My own experience echoes this: collaboration between process engineers, toxicologists, and sustainability managers sets the stage for real breakthroughs. Seeing the compound’s journey from a lab curiosity to a linchpin of everyday nutrition shows that a single molecule can move mountains, provided innovation keeps pace with public need and responsible stewardship.
Vitamin E is one of those nutrients you see everywhere, right next to vitamin C and calcium on your multivitamin bottle. You might not think twice about how it ends up there, but there’s science, chemical expertise, and a fair bit of global trade making sure you get enough. The main ingredient powering large-scale vitamin E production is 2,3,5-Trimethylhydroquinone. This chemical compound might not get any headlines, but it plays a crucial role in health and nutrition sectors. Factories mix it with isophytol to synthesize vitamin E on an industrial scale.
Not everyone gets enough vitamin E from diet alone. The jump in demand for fortified foods, supplements, and animal feed has put extra pressure on producers to deliver more, faster, and cheaper. 2,3,5-Trimethylhydroquinone stands out for efficiency, purity, and cost. Factories can crank out hundreds of thousands of kilos a year, keeping shelves stocked and prices steady. Reliable vitamin E also means stable dairy, poultry, and meat production. Animals need nutrients in their feed for healthy growth, and that trickles down to everything from your breakfast eggs to a block of cheese.
Some supplement buyers worry about where their nutrients come from. Most manufacturers work under strict standards, since 2,3,5-Trimethylhydroquinone must meet high purity to avoid contaminants ending up in final products. Problems can arise if quality slips—fake or contaminated vitamin E recalls have made global news. With so many chemical steps between raw material and capsule, attention to chemical sourcing and safety makes a difference. I’ve watched consumer trust evaporate overnight after a supplement scare, but clear, traceable raw materials go a long way in rebuilding that trust.
Synthesizing 2,3,5-Trimethylhydroquinone draws on petrochemical feedstock, which raises tough environmental questions. Disposal of chemical waste and use of non-renewable ingredients worry environmental groups. It’s tough to balance affordable nutrition for billions and responsible chemical manufacturing. Greener chemistry methods, tighter regulations, and transparent audits offer a path forward, even if the transition brings cost and complexity. Some companies experiment with bio-based or enzymatic routes to trim environmental impact, but fewer breakthroughs hit large scale. Sourcing green raw materials costs more but fits a growing demand for eco-friendly supplements.
Europe and Asia drive most of the world’s vitamin E production, and their labs invest in safer, cleaner chemical steps. Real movement happens not with grand promises but with steady, practical changes: new catalysts that cut down on byproducts, recovery systems for solvents, and local partnerships to reduce shipping and storage risks. If shoppers demand cleaner supplements, companies follow—or risk falling behind. Automation, better supply chains, and stricter lab testing all protect buyers and boost confidence.
Anyone who takes a multivitamin, uses fortified cereal, or relies on steady animal feed has 2,3,5-Trimethylhydroquinone somewhere in their supply chain. The chemical itself isn’t found in your breakfast or medicine cabinet, but without it, the vitamin E market would look very different. In my own work, I’ve seen small changes at the raw material level ripple out to major shifts for end users. Paying attention to the source and impact of these materials isn’t a luxury or a niche concern—it’s essential for better health and a cleaner planet.
2,3,5-Trimethylhydroquinone holds a special place for anyone who’s spent hours hunched over a lab bench. This organic compound shows up as a colorless to pale yellow solid, something you’ll notice as soon as you open a bottle. Its structure resembles hydroquinone, but with three extra methyl groups at strategic spots—those tweaks make more difference than many realize.
The texture of 2,3,5-Trimethylhydroquinone often reminds me of fine crystals or a powder. It doesn’t flow quite like table salt, but sift a little through your fingers, and you’ll spot those fine grains clinging together. Tossing a pinch onto a watch glass under bright light, you’ll get a subtle sheen—nothing as flashy as table sugar, more subdued, easy to overlook if you aren’t paying attention.
Not many people will ever notice the smell, but if you spend enough time around phenols, you’ll recognize a faintly medicinal odor, something reminiscent of other quinones but softened by those methyl groups. As for taste, the compound never makes it near food in a responsible lab, for good reason—its bitterness is matched only by its chemical potency.
Heat this substance gently and you’ll see it melts at about 168 to 172°C. Push the temperature higher, and it boils around 280°C before breaking down, so it doesn’t travel well in vapor form. Unlike some hydroquinone relatives, it holds up nicely in dry storage, but introduce moisture or air and you’ll watch it start to oxidize, turning darker with time.
Chemically, 2,3,5-Trimethylhydroquinone keeps things interesting. The molecular formula, C9H12O2, means three methyl groups are nestled onto the benzene ring. These groups don’t just add bulk—they tweak how it reacts. That extra methyl protection makes this compound stand up to mild oxidizers better than plain hydroquinone.
In my experience, most folks working in synthesis value this stability. In reactions, you’ll find it behaves much like other dihydroxybenzenes, trading its electrons willingly but with a touch more selectivity. It can oxidize to 2,3,5-trimethylbenzoquinone if given the right push—excellent if you need a reliable redox reaction in your toolkit.
Mix 2,3,5-Trimethylhydroquinone with strong acids or bases, and you get the usual array of phenolic tricks: color changes and, eventually, breakdown products if you push too hard. It dissolves best in organic solvents like ether or ethanol, but water only grudgingly takes up a little. That solubility gap matters in both lab procedures and downstream processing, forcing anyone handling it to rethink solvent use and waste disposal.
Knowing these quirks matters far beyond the textbook. I’ve watched projects get stuck because someone underestimated its oxidative resistance. That same trait helps it function as an antioxidant, showing up in vitamin E manufacturing and stabilizing formulations that would otherwise spoil quickly. Without understanding its physical handling—tendency to oxidize, stubborn solubility—costs and contamination risks pile up.
For those making or working with pharmaceuticals, dyes, or even specialty polymers, these chemical and physical features drive how you design each step. Safe storage, clear protocols for handling byproducts and a good understanding of redox chemistry make the difference between lab success and disaster.
Every time I pick up a bottle with a complex name, my instinct is to find the safety sheet before the cap comes off. 2,3,5-Trimethylhydroquinone looks low-key at a glance—white crystals, mild scent, not as notorious as some lab hazards. That mild appearance can trick people into skipping necessary precautions. Touching, inhaling, or even storing it the wrong way can cause skin irritation, eye trouble, and headaches at the very least. This compound sometimes finds its way into vitamin E synthesis and research labs. Proper handling isn’t just a tick-box item from the safety manual. If you treat it casually, you’re risking your own comfort, maybe even your long-term health.
I’ve watched new lab techs underestimate how quickly a mishap unfolds. I always wear gloves, splash-resistant goggles, and a lab coat. If someone says it’s overkill, they haven’t felt burning eyes or itchy wrists. Keep this compound in the fume hood. Breathing its dust or vapors for a few minutes can leave you woozy or coughing for the rest of the day. Some labs use personal air monitors in smaller, older rooms to catch low-level exposure early. It’s not paranoia—it’s protection.
If there’s a spill, sweeping up dry powder creates more airborne particles than cleaning up a liquid spill. Use a HEPA-filtered vacuum or wet methods with absorbent pads. Toss any materials you use right into a hazardous waste bin. At my old job, a careless sweep sent dust into an adjacent workspace, leading to cross-contamination and extra hours spent cleaning and monitoring. Good recordkeeping on incidents cuts down on future repeats.
At a glance, storage seems like the simple part of handling chemicals. 2,3,5-Trimethylhydroquinone likes cool, dry rooms with lots of ventilation. Too much warmth or exposure to sunlight speeds up degradation, leading to byproducts you don’t want to discover after the fact. I once cracked open a container left too long in a warm storeroom. The smell and yellow crust said it all—the batch had turned, and disposal became a new headache.
Tightly sealed, clearly labeled containers save a lot of confusion down the road. I’ve seen labels fade, fall off, or get covered in dust. Take two minutes to update them or add a backup tag. Segregate storage away from oxidizers and acids. Even with a fire-resistant cabinet, storing reactive chemicals too close invites disaster. Overflow and overstocking set the stage for forgotten supplies. With these types of chemicals, knowing what’s on hand, and where, usually saves money as well as hassle.
The internet overflows with safety checklists, but real protection comes from steady, simple habits. Remind everyone, even the veterans, that shortcuts lead to accidents. Use good extraction systems and encourage mandatory glove and goggle use on every shift, not just during inspections. Having spill kits and washing stations in easy reach eliminates scramble time during emergencies.
A culture of clear instructions, routine spot checks, and respectful communication discourages carelessness. If someone makes a mistake, openness about what happened helps everyone improve. In the end, 2,3,5-Trimethylhydroquinone won’t bow to laboratory experience or fancy credentials—just practical care and a respect for proven routines keep outcomes predictable and people healthy.
2,3,5-Trimethylhydroquinone shows up in the world of chemistry with a formula: C9H12O2. This molecule holds a molecular weight of 152.19 g/mol. It isn’t just a jumble of numbers and letters—this compound plays a pivotal role in the production of vitamin E, which fuels a chunk of today’s nutrition and health industries.
Plenty of people walk past the word hydroquinone and never look back, but if you’ve ever read up on vitamin E, you may have stumbled onto it. In my time chatting with supplement formulators and production chemists, I’ve seen how this chemical ends up as a building block in vitamin E synthesis. It’s not the shiny finished product you find on health store shelves. Instead, it acts as a foundation that ties the process together. Without it, making dl-alpha-tocopherol—a form of vitamin E—would come down to a much more expensive or inefficient game.
You might wonder what makes C9H12O2 special. Those three methyl groups tucked onto the benzene ring adjust the chemical’s behavior. They tweak its reactivity, making it easier to steer the intermediate toward becoming vitamin E. Unlike plain hydroquinone, these methyl groups keep the chemistry cost-effective and scalable. In the world of industrial production, every tweak counts.
Quality remains on everyone’s mind, especially with the rigorous standards in pharma and nutrition spaces. I’ve spoken to analysts who run entire quality-control departments. For them, the tiniest impurity can set off alarm bells, risking finished batches of supplements. Using a well-characterized intermediate like 2,3,5-Trimethylhydroquinone makes it possible to keep those standards tight. Sourcing this intermediate from reliable producers with transparent records and strong track records directly impacts trust and safety for everyone down the line, from manufacturer to consumer. Reputable suppliers often back their batches with transparent third-party testing results, helping to maintain integrity throughout the supply chain.
Manufacturing chemicals at this scale brings some thorny trade-offs. Factories generating 2,3,5-Trimethylhydroquinone must manage waste properly. Over the years, I’ve seen regulatory eyes zoom in on improper solvent disposal and workplace safety lapses. Strong oversight and environmental checks keep risks in check, but for true improvement, facilities must invest in newer, greener chemistries. Easier said than done, but the shift toward catalyst recycling, safer reagents, and cleaner emissions doesn’t just tick boxes—it keeps neighborhoods healthy and sets an example for the wider industry.
More companies want to shrink their environmental footprints. As vitamin E’s popularity grows, I keep an eye on biotech startups experimenting with engineered microbes that might one day replace chemical production lines. In the meantime, understanding the molecular backbone—like 2,3,5-Trimethylhydroquinone—helps consumers and producers make informed choices. Facts like its chemical formula and molecular weight are more than academic trivia. They serve as a small but vital piece of a much bigger puzzle that connects science, health, and sustainability.
Every time someone swallows a vitamin E pill, there’s a good chance they benefit from work that happens in the background inside chemical plants. 2,3,5-Trimethylhydroquinone, or TMHQ as folks in the lab often call it, forms the backbone of making vitamin E. This chemical shows how something that looks obscure on paper plays a huge part in real health outcomes.
TMHQ doesn’t exist in nature in large amounts, so it comes out of smart chemistry. The story usually begins with m-cresol, a raw material that comes from coal tar or petroleum. Factories use Friedel-Crafts alkylation to add extra methyl groups to m-cresol. Then, they carry out oxidation with agents like potassium persulfate or chromium trioxide to get the quinone structure. Hydrogenation steps follow, using catalysts such as palladium on carbon. Each process step brings its own fingerprint of expense, waste, and technical headaches.
I’ve spoken with chemical engineers who wrangle with balancing yields, safety, and byproducts. They need reliable suppliers for raw ingredients, tight thermal control for reactions, and robust protocols for purification. Even small errors can lead to impurities which, if left unchecked, compromise the quality of downstream vitamin E. In a global market, these standards matter. Regulators in each country keep an eye on what’s inside the bottle, since the stakes touch human health.
Manufacturing TMHQ isn’t gentle on the environment, especially if older technology stays in use. The oxidation steps can generate lots of chemical waste. Certain oxidants, like chromates, raise health flags for workers and local water supplies. Not every company puts the same weight on cleaner methods. Some producers invest in closed-loop recycling or swap in less hazardous oxidants, but not enough are on board.
I’ve seen places where robust ventilation systems and skilled operators keep risks low, and I’ve seen others where corners get cut, endangering people and polluting neighborhoods. Tougher regulations and real enforcement help, but the chemistry also needs tuning to reduce waste at the source. Green chemistry approaches—like using oxygen from air for oxidation or adopting safer catalysts—hold promise. If the sector shifts, everyone sharpens their game out of necessity.
TMHQ synthesis plugs into a global network. China, India, and Western Europe all host major sites. If a plant goes down or trade flows get choked, vitamin E prices shoot up and supplement makers scramble. Covid-19 showed how fragile these chains get, sparking talk about redundancy and onshoring. Responsible manufacturing doesn’t just lower risk for the local community; it backs up global health systems against future shocks.
Investing in smarter synthesis isn’t just about lowering costs or boosting purity; it directly affects sustainability for the whole supply chain. Manufacturers looking forward recognize this and push for innovation in process chemistry.
TMHQ reminds us that real-world chemistry hinges on technical skill, environmental responsibility, and transparency in sourcing. New routes using biocatalysts or electrochemistry, now under development in some research groups, point toward cleaner and more resilient production. Rather than sticking with what’s familiar, the industry faces a clear path: back the chemists and engineers pioneering new approaches, and demand that the end product stays safe—from factory floor to medicine cabinet.
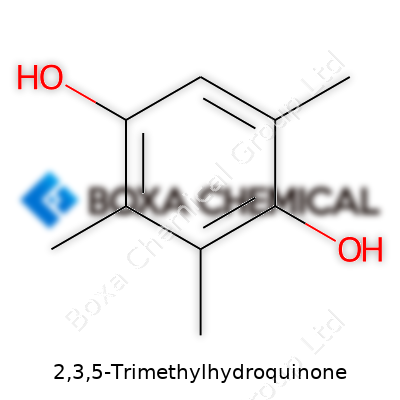
| Names | |
| Preferred IUPAC name | 2,3,5-Trimethylbenzene-1,4-diol |
| Other names |
2,3,5-TMHQ 2,3,5-Trimethyl-1,4-benzenediol |
| Pronunciation | /ˌtraɪˌmɛθəlˌhaɪdrəkwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | 700-13-0 |
| Beilstein Reference | 1209226 |
| ChEBI | CHEBI:32398 |
| ChEMBL | CHEMBL16333 |
| ChemSpider | 207835 |
| DrugBank | DB03849 |
| ECHA InfoCard | 13b037b2-f7d9-4854-84e1-9f8beec9b216 |
| EC Number | 205-237-5 |
| Gmelin Reference | 104896 |
| KEGG | C06533 |
| MeSH | D011210 |
| PubChem CID | 6973 |
| RTECS number | GV5950000 |
| UNII | V98OOO4X9K |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID5039707 |
| Properties | |
| Chemical formula | C9H12O2 |
| Molar mass | 166.22 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.12 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 2.38 |
| Vapor pressure | 0.00225 mmHg (25°C) |
| Acidity (pKa) | 11.0 |
| Basicity (pKb) | pKb = 10.61 |
| Magnetic susceptibility (χ) | -55.0e-6 cm³/mol |
| Refractive index (nD) | 1.541 |
| Viscosity | 15 mPa·s (25 °C) |
| Dipole moment | 2.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 208.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –276.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1582 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A11CC04 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 79 °C |
| Autoignition temperature | 410 °C (770 °F) |
| Lethal dose or concentration | LD50 oral rat 1670 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 3900 mg/kg |
| NIOSH | WQ8750000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | REL: 2 mg/m³ |
| IDLH (Immediate danger) | Unknown |