Chemists first documented 1,4-naphthoquinone in the mid-19th century, driven by curiosity about naphthalene derivatives. The early days of organic synthesis focused a spotlight on aromatic hydrocarbons like naphthalene, extracted from coal tar during the rapid industrial growth in Europe. Pioneers in organic chemistry managed to oxidize naphthalene, unlocking this yellow crystalline compound and opening new lines of inquiry into its chemistry and utility. From the late 1800s, researchers turned their attention to both the basic science and the practical uses of naphthoquinones, building the foundation for later discoveries in dye manufacturing, pharmaceuticals, and agrochemicals. The compound’s story follows the twisting path of chemical research, where tinkerers and scientists alike have chased after new structures, not only to understand them but also to put them to work in the world.
1,4-Naphthoquinone features as a small molecule but packs a notable punch across the chemical and pharmaceutical industries. Companies manufacture it in bulk for use in intermediates, dye precursors, fungicides, and pharmaceuticals. Its commercial forms show up as a bright yellow crystalline powder, typically packed in airtight containers to prevent unwanted reactions with air or moisture. Naphthoquinone’s core business value has long rested on a reliable track record and consistent properties, earning trust among procurement teams that respond to ever-shifting regulatory and quality demands.
This molecule comes with a distinct sharp odor that’s hard to forget after a stint in a lab. It melts around 125-130 °C and boils upwards of 295 °C, which places some practical limits on its applications. It dissolves sparingly in water but mixes far more willingly with organic solvents such as ether, benzene, and alcohol. Its structure—two conjugated carbonyl groups attached to a naphthalene ring—serves up remarkable reactivity that both helps and hinders chemists, depending on the situation. The molecule’s yellow color arises from light absorption linked to these carbonyl groups, providing a visible fingerprint that makes identification straightforward.
Suppliers tend to uphold tight standards for purity, usually topping 98% by high-performance liquid chromatography. Technical data sheets detail not just melting point stability but also trace metal content, moisture levels, and residual solvents. Anyone handling shipments of this compound watches for hazard symbols that warn of skin and respiratory irritation or environmental hazards if spilled. Correct labeling lists the United Nations number (UN 2811), hazard codes, and supplier contact details. Keeping thorough documentation helps teams track compliance with REACH, OSHA, and local safety regulations.
Traditional routes for preparing 1,4-naphthoquinone start with direct oxidation of naphthalene, most often using chromic acid or other strong oxidants. Some manufacturers favor air oxidation in the presence of a metal catalyst, a strategy that slashes hazardous waste and meets cleaner production standards. Many firms keep the focus on catalytic efficiency, scaling up processes to minimize cost and environmental impact. Each manufacturing run typically includes steps for extraction, recrystallization, and thorough drying to reach high-purity benchmarks.
The molecule’s dual carbonyl groups drive a broad range of chemical transformations. Nucleophilic additions can produce various hydroxylated derivatives, which pharmaceutical researchers regard as crucial scaffolds for drug candidates. Synthetic chemists pursue halogenation, amination, and reduction reactions that open the door to tailor-made functionalized compounds. These transformations fuel the creation of new dyes, pigments, and agrochemicals, reflecting the versatility of the naphthoquinone core. Its strong electrophilicity provides an excellent starting point for many exploratory organometallic reactions as well.
Throughout documents, 1,4-naphthoquinone appears under several names: para-naphthoquinone, quinone A, and sometimes simply naphthoquinone. In chemical catalogs, it’s matched to the CAS number 130-15-4. For regulatory or trade purposes, labeling often includes these synonyms to ensure clarity across borders and supply chains.
Working with 1,4-naphthoquinone calls for meaningful safety measures. Exposure can lead to skin rashes, eye irritation, or breathing difficulty, based on documented workplace cases. Personal protective equipment ranks high on protocols, including gloves, goggles, and well-ventilated fume hoods. Storage spaces require reliable air control and strict fire precautions, as organic dust and vapors from this compound still count as fire hazards. Proper training lays the foundation for risk management, along with access to updated safety data sheets. Spills need swift cleanup with absorbent materials and prompt disposal according to hazardous waste rules. I’ve seen firsthand that even in well-regulated labs, a lapse in attention spells trouble with chemicals like this.
Different industries look to 1,4-naphthoquinone for varied reasons. Dye makers appreciate its use as a feedstock for vat dyes and pigment formulations. Crop science teams depend on derivatives such as phyton and plumbagin for plant protection products. The pharmaceutical sector relies on related naphthoquinones in antimalarial drugs, topical antimicrobials, and certain antitumor compounds. Material scientists experiment with it as an electron acceptor in organic solar cells and battery research. Experience in industry tells me that companies value predictable performance and supply, spurring closer partnerships between chemists and end-users to capture new applications.
Academic labs and corporate R&D divisions continue to scout fresh derivatives of 1,4-naphthoquinone in pursuit of new therapies and sustainable technologies. Recent studies home in on its redox properties, chasing leads in energy storage for organic batteries and supercapacitors. Medicinal chemists probe structural variations to hit stubborn bacterial infections and drug-resistant cancers, drawing inspiration from natural naphthoquinone analogs like lapachol and juglone. I’ve watched researchers light up over a new molecule’s promise, only to circle back for another round of synthesis days later—evidence that the field thrives on persistence and curiosity.
Long-term safety has taken an important seat in regulatory reviews. 1,4-naphthoquinone’s potential to cause oxidative stress and DNA damage has attracted detailed toxicology work in cells and animal models. Data points to the risks of skin and mucous membrane irritation, and animal studies indicate potential organ toxicity following sustained exposure. These findings have pressed regulatory bodies to demand safe handling protocols and to monitor workplace exposure more closely. The blend of recognized utility and genuine risks means both industry and regulators face tough decisions on permissible exposure levels and labeling. Teams handling this compound regularly put special effort into minimizing unnecessary contact and tracking signs of overexposure.
Looking ahead, the story of 1,4-naphthoquinone still has fresh chapters waiting to be written. Energy researchers in particular see promise in harnessing its redox flexibility for organic flow batteries and next-gen photovoltaics. More biologists test its role in managing plant disease, spurred by public demand for green agrochemicals. Pharmaceutical pipelines continue to tap naphthoquinone-based molecules for new mechanisms of action against hard-to-treat cancers. Industry veterans often discuss the tension between utility and hazard; so, safer synthetic approaches and downstream products will drive coming innovations. As markets embrace sustainability and safety, forward-thinking chemists and production teams prepare to adjust both process and product for the long haul.
1,4-Naphthoquinone looks fairly simple at the molecular level, but its influence stretches far. Its bright yellow color might catch your eye, but chemists care more about the way it behaves—reactive, handy, and a building block for many other molecules. My own early days in the lab started with compounds like this, and I’ve seen first-hand how a single substance can play a role in everything from making dyes to stopping fungus in its tracks.
Street signs, T-shirts, and even school backpacks often owe their bold colors to molecules like 1,4-Naphthoquinone. The textile and printing industries lean on derivatives such as lawsone (from henna) to create deep reds and browns. Synthetic pigments count on the stable color it delivers, and some of the world’s brightest, lasting dyes trace their chemical roots back to it. You might not realize it, but chances are you have handled paper or worn fabrics that started their color life with this molecule.
Pharmaceutical researchers constantly search for new ways to fight disease, and the quinone family doesn’t disappoint. 1,4-Naphthoquinone provides a foundation for anti-microbial, anti-inflammatory, and anti-tumor agents. Plumbagin, a close cousin, captures scientists’ attention for slowing down certain cancer cells. My pharmacy instructor once showed us studies tracing the history of chloroquine, used for malaria, back to the naphthoquinone structure. Antifungal creams, antiseptics, and some anti-parasitic drugs draw from the same well.
Lab workers favor 1,4-Naphthoquinone for more than just color and medicine. It acts as a versatile intermediate, letting chemists snap other atoms or groups onto it to create police dyes, insecticides, and specialty polymers. During my grad school rotations, I watched a process that used it to scavenge electrons in polymer chemistry—turning liquid resin into cured, solid parts. Chemical plants use it to manufacture hydrogen peroxide, and polyester fabrics often owe their gloss to reactions involving this compound.
No chemical comes without risks. 1,4-Naphthoquinone can irritate skin or lungs, especially in industrial settings. Workers handling it follow strict protocols, wearing gloves and masks. I remember the safety training videos—the message was always clear: use the minimum, avoid open containers, and never treat ventilation as an afterthought. Regulators set clear limits on workplace exposure to limit accidental harm.
Chemists seek greener methods that cut down on waste and hazard. Cleaner synthesis and better filtration help; several startups work on bio-based methods to make some naphthoquinone derivatives. University teams now look for ways to patch its chemical core onto new treatments for old diseases. Practical uses keep expanding, including as a sensor for environmental monitoring—detecting certain pollutants thanks to its rapid electron transfers.
From art to antibiotics, 1,4-Naphthoquinone has deeper roots than most people expect. Careful handling and clever research promise to push those roots even further.
1,4-Naphthoquinone is a mouthful to say, but a lot of chemists know it by its simpler name, para-naphthoquinone. I remember walking into a research lab at college and seeing that yellowish powder on a labeled jar. The warning stickers alone made me pause. Over time, the facts stuck with me: this compound acts aggressively on living tissues, and it pays to treat it with extra caution.
Scientists track chemical hazards using real cases, not just theoretical guesses. Several studies show that 1,4-Naphthoquinone can irritate skin and eyes on contact. If inhaled, it can trigger coughing and a sore throat, sometimes even damaging lung tissue with repeated exposure. These outcomes aren’t just lab curiosities. When mishandled, the risks can follow workers home after a shift.
Research done on laboratory animals points out the bigger dangers. Given in high enough doses, this compound has harmed organs like the liver and kidneys. It interacts with enzymes central to how cells breathe and function; messing with these processes often leads to tissue damage and inflammation. In the chemical industry, I’ve seen how proper safety protocols make a difference. One missed glove, one spill, and small exposures multiply into long-term problems.
This stuff doesn’t just vanish. When released into water or soil, it lingers, posing risks to aquatic life. Some fish and plant species can’t handle even trace-level contamination. For people living near manufacturing plants, this raises valid concerns. Public databases from the EPA and NIOSH back up these worries, listing 1,4-Naphthoquinone as toxic with clear recommendations for handling and disposal.
Too often, companies focus on productivity and ignore personal protection until a near-miss happens. In jobs where chemicals like this come up, simple changes save a lot of grief—ventilation, sealed containers, gloves that actually get worn instead of left in a drawer. It’s not just about fancy gear, though. Training makes the biggest difference. Workers who understand the risk make safer choices.
Regulations in countries like the United States and Germany already label 1,4-Naphthoquinone as hazardous. Still, loopholes or lack of enforcement mean that mistakes happen. Community advocacy makes a difference: when neighbors demand environmental monitoring and stronger safety rules, companies respond. I’ve seen how public reporting on chemical spills pressures firms to clean up their act. Reports from organizations such as the World Health Organization give people tools to speak up with facts, not just fears.
Most chemicals have useful sides, but safety comes first. People in the field and those living nearby deserve honest risk assessments and real protection. The goal isn’t to ban chemistry. It’s about putting health first, leaning on practical solutions, and making sure lessons from past mistakes stick around longer than the danger itself.
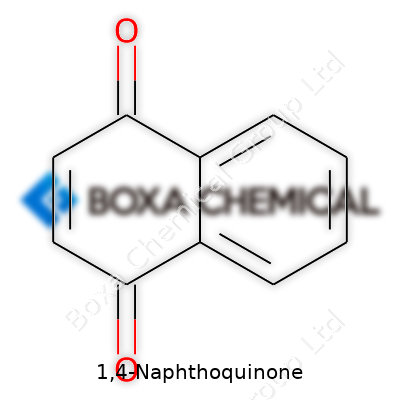
Step right into organic chemistry and you’ll bump into 1,4-naphthoquinone, a compound that quietly powers more than a few scientific corners. Think of it as a cousin of naphthalene, swapping out a couple of hydrogen atoms for oxygen atoms and winding up with a molecule that’s much more chemically active. In plain language, 1,4-naphthoquinone’s backbone comes from two fused benzene rings, just like naphthalene. Take carbons in places one and four, plug in oxygen atoms as carbonyl groups, and you’ve got its fingerprint.
Molecules like these pick up attention because of their flat structure — scientists call it planarity — making them helpful in redox chemistry. They perform in batteries, dyes, drugs, and even pesticides. That sort of reach is a clue about why this unassuming compound deserves a closer look beyond its textbook diagram.
The formula for 1,4-naphthoquinone reads C10H6O2. Chemists sketch two rings side by side; each ring shares a side with the next, giving ten carbons. Two of those carbons (positions one and four) hold double-bonded oxygens, forming quinone units. The rest gather around as part of an aromatic system, keeping the molecule stable but reactive in just the right spots.
If you look at it in three dimensions, it's flat as a pancake, which means it slides into other molecules smoothly. That planarity matters because it lets 1,4-naphthoquinone participate in electron transfer reactions, a key feature in biological roles and industrial uses alike.
Charts and models only tell half the story. Personal experience in a research lab taught me how essential compounds like 1,4-naphthoquinone become, especially when piecing together the puzzle of cellular respiration. It's part of the machinery that shuttles electrons in living cells. Certain bacteria rely on it as a go-between for accepting and handing off electrons. In medicine, this structure forms the backbone for several antifungal and anticancer drugs, proving how useful these simple tweaks to naphthalene can be.
And if you’ve used dyes, you’re probably looking at products indirectly touched by naphthoquinone chemistry. Its ability to cycle between oxidized and reduced forms makes it a workhorse in pigments and dyes. Plus, industries tap into its structure for pesticides because it’s effective at disrupting biological processes in unwanted pests.
With that range of uses comes a tangle of safety and environmental questions. Simple as it looks, this molecule can irritate skin and eyes, and when factory runoff contains quinone residues, it risks harming aquatic wildlife. It’s important for industries to treat waste streams and develop greener manufacturing steps. Green chemistry offers ways to produce naphthoquinone derivatives with less waste and softer reactions, swapping harsh conditions for catalysts and renewable feedstocks.
Lab work in college hammered home the importance of such steps. Spills aren’t just stains; they become environmental headaches. Science has set its sights on not just exploiting chemical properties, but managing the footprint they leave. Progress in this field comes from open conversations and constant tweaks to process and policy.
1,4-Naphthoquinone isn’t just a structure tucked away in a textbook; it shapes industries, medicines, and research across the world. I’ve seen its potential in academic and real-world settings. As research continues, tighter oversight of production and waste will keep this compound useful without unnecessary risk. Anyone working with chemicals, whether in a lab or on a factory floor, benefits from understanding both the structure and the impact of what’s in their hands.
I’ve spent enough time around small labs and warehouse storage to know that safe chemistry isn’t just for the textbooks. 1,4-Naphthoquinone, with its strong oxidizing properties and sharp yellow crystals, gets attention because it poses real safety risks. People handle it in chemical plants, academic labs, and sometimes in research spaces that aren’t as well-equipped as you'd hope. So it isn’t just about following a checklist; it’s about clear routines and respect for the compound.
This compound doesn’t tolerate carelessness with temperature or light. Storing it cool—ideally around room temperature, just below typical lab ranges—keeps its chemical structure stable and prevents dangerous decomposition reactions. Direct sunlight changes the chemical and sparks unwanted reactions. In my own experience, airtight amber bottles tucked away in ventilated, labeled cabinets prevent accidents and keep the air free from dust or particles that could contaminate the product. Oxygen exposure turns storage into a risk, making sealed containers an absolute must for longevity and safety.
Even low moisture can trigger a reaction with 1,4-Naphthoquinone. If there’s humidity in the room, the powder clumps and may start unwanted chemistry you’d rather avoid. Silica gel packs or automated dehumidifiers hold humidity in check. Every time you open the container, keep exposure short. That goes double if you work in a place where summer turns storage rooms into swamps. If you drop any of the powder, dry wipes and careful sweeping—not water—collects the mess while keeping things safe and dry.
Anyone who handles this compound should know the risks of skin exposure, inhalation, and eye contact. Gloves—nitrile or butyl are much better than latex—along with goggles, lab coats, and sometimes a face mask go a long way. Fume hoods protect your lungs, not just your reputation for following protocol. Regular training keeps teams sharp, not complacent. I’ve seen people lose focus and pay the price with minor burns or fumes in the nose. It’s always tempting to skip PPE for “just one quick step,” but the risks don’t care about shortcuts.
1,4-Naphthoquinone doesn’t play well with strong reducing agents. Accidentally mixing with easily oxidized materials, or letting it come near open flames, pushes it into the danger zone. Any storage solution keeps flammable substances out of the area and limits the risk by using steel cabinets that slow down fire and separate chemically reactive materials. Keep spill kits and small fire extinguishers close, so you never have to scramble if something does go wrong.
Safe storage and handling boil down to strong habits. Inventory logs track every gram of the substance, so nothing goes missing and nothing gets old past its prime. Shared spaces need checklists at arm’s length, with waste clearly marked and disposed of in sealed, approved containers. For people handling 1,4-Naphthoquinone every week, practical routines keep workplaces safer than any set of locked cabinets or posted warnings alone. Good chemistry is good safety, and good safety means never cutting corners.
1,4-Naphthoquinone pops up often in laboratories. Chemists value it for making dyes, pharmaceuticals, and as a precursor for a handful of chemical syntheses. Curiosity sometimes grows about where exactly to purchase it and how much a bottle will cost, especially for those starting out in research or trying to understand the supply chain on a practical level.
You won’t find this compound at the local hardware store. Most of the time, buyers look to chemical supply companies. Sigma-Aldrich, Alfa Aesar, and TCI America hold sway over much of the scientific market. Their catalogs list 1,4-Naphthoquinone in different quantities and grades, ranging from small research vials to bulk packaging suitable for industry.
Online platforms also play a big role. These include specialty sites like Fisher Scientific, Lab Alley, and ChemShuttle. Online marketplaces such as Alibaba or Made-in-China.com offer competitive prices but raise concerns about purity, shipping reliability, and legal importation. For most researchers, buying from a named scientific supplier brings peace of mind. Labs usually need to submit information proving legitimate use to complete an order.
There’s no single price for 1,4-Naphthoquinone. Like most chemicals, cost shifts depending on amount and purity. For high-purity powder (analytical grade), small bottles of 5 to 25 grams fetch anywhere from $40 to $120 through mainstream suppliers. Bulk requests bring the price per gram down, sometimes well below a dollar, but few outside manufacturing need that volume.
Shipping and dangerous goods surcharges add to the bill. These kinds of fine chemicals face tight regulations due to hazards if mishandled. It’s worth noting that quoting prices found online doesn’t always tell the whole story. Suppliers update catalogs, and chemical markets respond to global events—everything from factory shutdowns in China to new regulations push prices in unexpected ways.
Reliable access to 1,4-Naphthoquinone matters for research labs and small-scale manufacturers. It supports ongoing projects in organic synthesis, drug discovery, and basic chemical education. Chasing down high-purity chemicals can be frustrating without a trusted source. Less experienced researchers learn quickly that sticking with established suppliers reduces headaches. Unvetted sources sometimes ship contaminated chemicals or fail on delivery. Poor-quality reagents spoil experiments and can set a project back by weeks.
Clear legal pathways for buying chemicals like this support legitimate research while limiting risks from irresponsible handling. Regulations protecting the public, like Material Safety Data Sheet (MSDS) requirements and purchase documentation, can feel like stumbling blocks, but they serve a greater good. The challenge comes from balancing access with safety and keeping prices reasonable for non-corporate buyers.
Trust builds with direct experience. Ordering from a recognized distributor, verifying purity, and planning for safe storage goes far. New researchers should talk to colleagues or purchasing managers before placing an order. Price comparison works best over the phone or through customer service emails—catalog prices haven’t always caught up to deals or institution-specific pricing.
For anyone just wading into chemical procurement or working on research with tight budgets, patience and due diligence matter more than ever. The road from catalog to shelf isn’t always smooth, but making informed choices ensures the next experiment starts on solid ground.
| Names | |
| Preferred IUPAC name | naphthalene-1,4-dione |
| Pronunciation | /ˌwʌn.fɔːr næfˈθoʊ.kwɪ.noʊn/ |
| Identifiers | |
| CAS Number | 130-15-4 |
| Beilstein Reference | 1209227 |
| ChEBI | CHEBI:48161 |
| ChEMBL | CHEMBL1569 |
| ChemSpider | 7865 |
| DrugBank | DB03793 |
| ECHA InfoCard | ECHA InfoCard: 100.003.042 |
| EC Number | 1.6.5.2 |
| Gmelin Reference | 64162 |
| KEGG | C08197 |
| MeSH | D015907 |
| PubChem CID | 8650 |
| RTECS number | QC6300000 |
| UNII | DSG6T49U1S |
| UN number | UN2662 |
| Properties | |
| Chemical formula | C10H6O2 |
| Molar mass | 158.15 g/mol |
| Appearance | Yellow crystalline powder |
| Odor | pungent |
| Density | 1.318 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.67 |
| Vapor pressure | 0.005 mmHg (25 °C) |
| Acidity (pKa) | pKa = 4.09 |
| Basicity (pKb) | 8.34 |
| Magnetic susceptibility (χ) | -54.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.617 |
| Viscosity | 13.7 mPa·s (at 50 °C) |
| Dipole moment | 2.01 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 122.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -50.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -296 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | D05AC04 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 1, Special: - |
| Flash point | 113°C |
| Autoignition temperature | 533 °C (991 °F; 806 K) |
| Explosive limits | 4 to 24% |
| Lethal dose or concentration | LD50 oral rat 200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 190 mg/kg (oral, rat) |
| NIOSH | NA9575 |
| PEL (Permissible) | PEL: 0.78 mg/m3 (OSHA) |
| REL (Recommended) | 0.0005 |
| IDLH (Immediate danger) | IDLH: 25 mg/m3 |