People started isolating 1,4-Benzoquinone back in the early nineteenth century, long before chemists had the analytical tools we see in today’s labs. It didn’t take long for researchers to realize its strong oxidizing character. That defining feature made it stand out from the aromatic compounds it was often confused with. The naming, both systematic and regional, has shifted through the years, but the compound kept drawing attention for its ability to shuttle electrons in redox reactions. Whether found as a natural byproduct in plants or synthesized in the lab, its bright yellow hue marked it out as special enough for early chemists to take note and dig deeper. Many students recall their first organic chemistry course, learning about quinones both through lab experiments and as classic textbook examples of electrophilic compounds.
Today, 1,4-Benzoquinone shows up packaged in glass containers, easily mistaken for other crystalline powders on a chemical shelf. Also known as para-benzoquinone, this compound remains a staple for both industrial and research settings, with systematic rebranding by suppliers who target specific uses. Whether used as an intermediate in dye synthesis or as a redox mediator in electronics, the material keeps occupying a central spot for chemists and process engineers alike. In every use case I’ve encountered, people seem to focus on its dual talent: strong reactivity paired with pretty straightforward handling, given clear knowledge of safety measures.
At room temperature, pure 1,4-Benzoquinone takes the form of a yellow, needle-like solid with a distinctive sharp odor that reminds some chemists of bleaching agents. It melts just above 115°C, boiling at 289°C, releasing pungent vapors at moderate heating. Exposure to air and light quickly decomposes or darkens it, hinting at its underlying chemical drive. Its solubility in organic solvents like alcohol and ether makes it versatile, while its rapid reduction under mild conditions to hydroquinone reflects just how reactive the quinone ring can be in the right hands. This reactivity, thanks to the electron-deficient double bonds and carbonyl groups, defines the way researchers and process chemists approach its storage and use.
Vendors provide 1,4-Benzoquinone in purities above 98%, with labeling requirements dictated by chemical safety laws in the country of sale. The product label lists CAS number 106-51-4, sometimes adding “para-quinone” for clarity. Gross impurity levels, percent water content, and recommended storage conditions fill out most product sheets. These details matter in the lab, especially for colleagues working in pharmaceuticals or electronics, where technical consistency drives yield and regulatory compliance. I’ve seen first-hand that even minor contamination can throw off downstream synthesis, leading teams to favor reputable suppliers who show complete batch certification and transparent sourcing.
Traditionally, chemists produce 1,4-Benzoquinone by oxidizing hydroquinone with strong oxidants, like ferric chloride or silver oxide. Modern industry favors catalytic or electrochemical oxidation, mostly for greener profiles and better scalability. Some processes slip in copper-catalyzed air oxidation under controlled heat, cutting down on waste and reducing worker exposure to hazardous reagents. My colleagues and I have debated the trade-offs between throughput and environmental footprint, but it’s clear that the search for scalable, less hazardous methods continues to shape process development. Small labs might still use textbook methods, but larger plants seek out processes with fewer side reactions and easy recovery systems for reagents and solvents.
1,4-Benzoquinone turns up in a range of transformations thanks to those activated double bonds and carbonyls. People routinely use it as a mild oxidizer, stepping in for harsher reagents when selectivity matters. Reduction to hydroquinone can be managed with simple hydrogen donors under mild conditions, while more complex modifications, like creating hydroxy or amino-quinone derivatives, build out new dye and pharmaceutical options. In my own work, I’ve seen it as a go-to mediator in organic electrosynthesis and as an electron acceptor in photochemistry. Others extend its chemistry with alkylation, halogenation, or metal coordination complexes, each route unlocking a new corner of applied research. The compound’s reactivity, though robust, demands careful stoichiometry – a little excess or a misjudged solvent swap can undo days of planning.
Over the decades, 1,4-Benzoquinone picked up a handful of names: para-benzoquinone, p-quinone, and simply quinone (the latter can be ambiguous in some regions). Chemical catalogues sometimes list it by its systematic IUPAC name, but for bench chemists and industrial buyers, “para-benzoquinone” usually wins out. Each name reflects a different part of its history, drifting from its roots as a niche reagent to a mainstream intermediate. In product management meetings, the choice of name can affect international shipping and regulatory filing, so suppliers often echo ‘p-benzoquinone’ for clarity across borders.
Labs and warehouses enforce strict protocols with 1,4-Benzoquinone. Prolonged inhalation or skin contact can trigger irritation, respiratory issues, and, with chronic exposure, even more serious health effects. Safety data sheets spell out the PPE: gloves, goggles, ample ventilation, and sometimes full-face respiratory gear for large-scale handling. Environmental release rules require closed systems, spill containment, and waste neutralization. Over the years, I’ve seen facilities move from open-bench manipulation to glove boxes and fume hoods, cutting down accidental exposure and protecting both people and the environment from cumulative low-level contamination.
Industries keep reaching for 1,4-Benzoquinone for reasons that range from simple to highly engineered. In dyes and pigments, it lays the groundwork for quinone-based colorants seen in textiles. In polymer production, it functions as both an oxidizer and a stabilizer. Pharma companies use it in targeted syntheses, feeding into antimalarial and anticancer compound development. Analytical labs include it in tests for unsaturation and as a mediator in electrochemistry. I’ve noticed that energy storage research, especially organic battery design, increasingly sparks interest in quinone derivatives, betting on their redox capacity as both cathode and anode materials for new battery chemistries. The compound’s presence in new tech reminders carries echoes of its old-school applications, bridging decades of chemical discovery.
The last decade brought a flood of new research, much of it focused on sustainable oxidation techniques and new applications in organic electronics. Chemists strive for lower-waste syntheses, using selective catalysts and greener solvents. A lot of the new patents involve integration in small molecule semiconductors, conductive inks, and as electroactive scaffolds for next-generation sensors. Pharmaceutical research circles back to the core structure, tweaking substituents to uncover new antimicrobial and antitumor activities. With the push toward renewable energy and biodegradable electronics, R&D teams chase after modified quinones for better battery performance and more resilient charge mediators. It’s hard not to get swept up in the curiosity-driven culture, watching basic building blocks like 1,4-Benzoquinone tie together so many areas of science and technology.
Decades of toxicity studies point to both acute and chronic risks tied to 1,4-Benzoquinone, especially for workers in production environments. Animal studies outline its oxidative stress pathways, with emphasis on liver and kidney impacts at high doses. Chronic exposure, even at low levels, raises concern for genetic toxicity and potential carcinogenicity, although regulatory agencies differ in their risk assessments. Modern safety training leans on molecular-level knowledge to set exposure guidelines, and regulatory updates now demand real-time monitoring, improved containment, and medical screening. In my experience, health and safety officers keep updating procedures as new data emerges, reflecting respect for both established evidence and evolving regulatory standards.
Looking ahead, 1,4-Benzoquinone wears many hats across chemistry and new materials science. Next-gen applications call for even higher purity, new derivatives, and production routes that reconcile efficiency with lower environmental tolls. In energy storage alone, quinone-based flow batteries edge toward commercial reality. Medical research circles closer to targeted therapies that exploit the redox cycling properties of the compound’s structural core. As sustainability pressures ramp up, the quest to minimize toxic byproducts, maximize recyclability, and harness redundancy in synthetic pathways grows louder. My own perspective is that the backbone of 1,4-Benzoquinone chemistry isn’t close to running out of opportunities, with each incremental improvement unlocking possibilities that didn’t exist a generation ago.
1,4-Benzoquinone, more often just called para-benzoquinone, shows up in far more places than people expect. It's not a household name, but in the world of chemical manufacturing, it plays a front-line role. Most folks working in synthetic chemistry know benzoquinone as a dependable oxidizing agent. It can quickly strip electrons from other molecules, which comes in handy during organic reactions. Over the years, I’ve seen it routinely put to work making dyes, certain pharmaceuticals, and perfumes. Projects that call for strong, reliable oxidizers often turn to 1,4-benzoquinone because it gets the job done without much fuss.
Dye makers rely heavily on 1,4-benzoquinone to produce vivid, lasting colors. The clean, fast reaction it provides helps in building both synthetic dyes and some pigments for textiles and plastics. It’s also mixed in the process for leuco vat dyes, which go onto cloth and then develop their colors when exposed to air or treated with other chemicals. Many colors people see in summer T-shirts and vivid plastic containers trace back, at least in part, to this single-molecule catalyst.
Pharmaceutical chemistry often revolves around clever rearrangements and careful control over functional groups. 1,4-benzoquinone serves as a crucial intermediate here, helping bring together larger and more complex molecules. Some cancer drugs owe their existence to pathways involving this little yellow solid. I've watched contract synthesis labs use it to put together the core of important antibiotic compounds and antiviral agents. Agrochemical companies also draw on benzoquinone to piece together certain insecticides and herbicides. The versatility here comes from both its reactivity and relatively low cost compared to flashier specialty chemicals.
Natural and synthetic rubbers need toughening agents and stabilizers to last longer. 1,4-Benzoquinone enters the mix to prevent premature hardening or rotting, especially during storage. The ability to slow down unwanted polymerization keeps products flexible and usable. This benefit cuts waste and reduces replacement rates for everything from tires to industrial seals.
Many research labs keep a bottle of benzoquinone tucked on a shelf. I remember learning how useful it could be in redox titrations and electron-transfer studies. University labs often use it as a benchmark or model compound. In undergraduate teaching, students quickly see the stark yellow of benzoquinone change as it reduces, offering a clear, visual sense of how chemical reactions unfold. Its reliability and straightforward chemistry make it a lab favorite.
Every industrial player using benzoquinone has safety protocols in place, and for good reason. It can irritate skin, eyes, and lungs, so handling calls for respect and proper gear. Moves towards greener chemistry and sustainability push research into finding alternatives or refining processes that generate less waste. While no perfect substitutes have arrived yet, better recycling methods and safer synthesis routes cut down risks for workers and communities.
1,4-Benzoquinone’s presence in so many fields—from dyes and drugs to polymers and educational labs—shows its value isn't likely to fade soon. With good safety controls and ongoing R&D focused on green chemistry, it keeps supporting progress all along the industrial supply chain. As new needs pop up, the knowledge and experience built around this compound will shape better practices and smarter materials for years ahead.
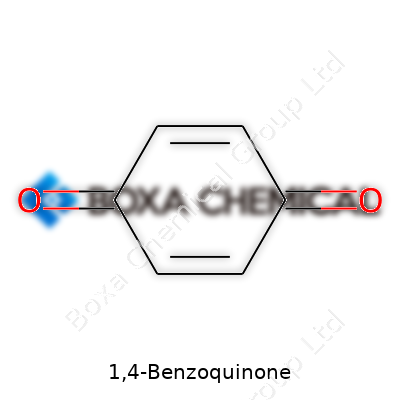
1,4-Benzoquinone comes with the simple yet powerful formula C6H4O2. Take a carbon ring with six carbon atoms, strip away the cloud of hydrogen that usually sits on aromatic rings, and plug in two oxygen atoms at the one and four positions. That’s benzoquinone – sharp, yellow, and not what most folks picture when thinking about “organic chemistry.” The structure breaks up into a six-membered ring with double bonds alternating through the ring. Toss in two double-bonded oxygen atoms, one on each end, and you’ve captured its skeletal look. This isn’t just drawing on paper – such features drive every reaction this compound gets involved in.
Straight facts: 1,4-Benzoquinone shifts the rules in organic reactions. Its dual carbonyl groups (those sharp double-bonded oxygens) draw electrons, making the molecule hungry for reactions, especially with nucleophiles. This means in industrial chemistry labs and classrooms alike, it acts as an oxidizing agent. Look at dyes, photographic chemicals, and even certain batteries—benzoquinone’s structure lets it cycle between oxidation states. From the Bayer process of producing hydrogen peroxide to redox flow batteries, its framework does the real lifting.
My first real taste of benzoquinone’s power came during a sophomore lab. Fiddling with a beaker of pale yellow crystals, we tried redox reactions. Mixing this stuff with a reducing agent made it snap into hydroquinone. It played out quickly, showing little patience for hesitation. I came to respect its bite—unlike more forgiving aromatic compounds, benzoquinone proved reactive and demanded respect for safety.
With those lively electrons come risks. Contact causes skin irritation, and inhaling the dust brings respiratory problems. You find safety data sheets larded with warnings; this isn’t overkill. From my own practice, proper ventilation, gloves, and goggles became routine, especially after watching a classmate suffer mild contact dermatitis. Larger spills or ventilation issues can push fumes into the room, emphasizing the need for good habits. In industrial settings, workers get regular health checks. The EPA tracks benzoquinone’s presence in chemical waste, tying it to best practices for disposal.
Factories discharge plenty of aromatic chemicals, benzoquinone included. Runoff finds streams and soil, where breakdown happens slowly. Filtering the waste streams with activated carbon helps, but costs pile up quickly. Community monitoring around chemical plants makes a difference, catching leaks before they turn into bigger headaches. Using more closed systems keeps handling safer, cutting the chance for exposure. Substituting less-harmful chemicals sometimes works, though benzoquinone’s reactivity often keeps it on the roster for key reactions.
Structural details aren’t just trivia. Knowing why carbonyl groups go where they do changes how people predict chemical behavior. 1,4-Benzoquinone stands out because its structure forces strong reactivity and offers flexibility to make valuable materials – from pigments and batteries to pharmaceuticals. Understanding it at a structural level, handling it with care, and developing safer ways to use or substitute it all spell out practical chemistry in action. That’s the story behind the formula C6H4O2 — it shapes far more than what most folks see in a textbook diagram.
1,4-Benzoquinone, sometimes known as para-benzoquinone or simply quinone, comes up in industries ranging from chemical manufacturing to photography. This yellow crystal has a pungent, strong odor and can be found in processes that create dyes, fungicides, and even rubber accelerators. On the surface, it seems like just another building block in the chemical world.
Despite its usefulness, quinone carries a reputation in laboratories for being pretty rough stuff. Breathing in its dust or vapor irritates the nose, throat, and lungs. Skin doesn’t fare much better—direct exposure burns or stains. Many workers remember the sting on their hands or a cough lingering after handling it, even with gloves or masks.
The evidence backing these effects isn’t hearsay. The Centers for Disease Control and Prevention and peer-reviewed toxicology studies point to its toxic nature. Swallowing a small amount isn’t just uncomfortable, it means stomach pain, headaches, or worse if a person has pre-existing issues. For folks working daily with benzoquinone, chronic exposure can lead to more than mild symptoms. Repeated skin contact sets off dermatitis, and inhalation potentially harms the kidneys and liver.
It’s easy to assume only factory workers have to worry, but accidents stretch beyond the industrial floor. Spills in storage rooms or poorly ventilated labs can bring anyone into contact with this chemical. Not every workplace keeps up with best practices, especially in older facilities with outdated ventilation or patchy safety gear. I’ve personally walked into smaller labs where simple gloves or face shields were missing from the bench—oversights that stack up over time.
Citing hard numbers, the Occupational Safety and Health Administration limits quinone exposure to 0.1 parts per million over an eight-hour period. Regulations like this come from hard lessons learned after previous lax standards left workers sick. The message rings clear: even tiny amounts can be dangerous, and there’s a real need for serious controls wherever this chemical shows up.
Better safety comes from a mix of practical choices. Engineers can keep exposures in check by installing hood systems and automating parts of production. Simple changes—like switching to closed containers or disposable pipettes—keep spills off skin and out of the air. Routine training keeps everyone alert to hazards, so forgetting eye protection or inspecting gloves becomes rare instead of routine.
For companies, investing in hazard reduction doesn’t just protect reputations, it also lowers the odds of lost productivity from health complaints. Replacing quinone with less toxic alternatives remains the gold standard, but as long as it stays common in industry, prevention stands as the real fix.
Community groups living near manufacturing sites also benefit from transparency. Facilities should publish information about what they store and release, so neighbors know what’s drifting from smokestacks. Strong regulation ensures companies clear up spills quickly, monitor emissions, and share data with independent groups. These efforts create trust, knowing that people aren’t being sidelined while risks are quietly managed out of sight.
Quinone’s hazards don’t make it evil, just something to be respected. Science gives plenty of evidence on its risks, backed by studies and regulatory action. With basic protective steps and community engagement, harm doesn’t become part of the cost of doing business. People working or living near places that use this chemical deserve nothing less.
Step into a lab where 1,4-Benzoquinone sits on the shelf. That sharp, irritating kerosene-like smell will tell you right away: this compound means business. I remember working late one night, watching a yellow crystal crust up on the edge of a beaker. Noticed a mistake right away—left the bottle open. Headache set in within minutes. Lessons like that stick. This isn’t just about following a protocol. It’s about looking out for yourself and your teammates.
This compound breaks down fast if it sees too much light or heat. Stores much longer in amber glass or steel, away from direct sunlight. High temperatures drive off vapors and speed up decomposition. At room temperature, pressure locked tight, spills and contamination don't sneak up as easily. Silica gel or a dry cabinet keep out the moisture. Every bottle on a lower shelf, not on top—less chance of a fall or sudden spill. Leakproof seals cut down on wasted product and possible exposure. I've seen sloppy sealing ruin more than a few shipments, causing headaches for weeks.
Labels matter. No one wants to grab a bottle thinking it’s something else. Use a bold, clear label, even when pulling out a working quantity. Sharpie fades over time—laminated, solvent-resistant tape works best. Hazmat symbols need to stand out. Seen more than one scare from a roommate mistaking it for a harmless dye.
Those fumes sting your nose and eyes for a reason. At higher concentrations, headaches or even lung irritation creep up. The right answer: handle benzoquinone in a proper hood with the sash down. The hum of the exhaust should feel like a safety net. No hood, no glove—walk away. Open containers mean trouble. Keeping a few activated charcoal canisters nearby can mop up lingering vapors. I’ll never forget a time the AC failed, and the smell stuck for hours—the cleaning team came in with proper filters and got it under control.
Lab coats, nitrile gloves, proper goggles—none of this costs much, but it keeps you out of the clinic. Benzoquinone stains skin yellow, dries it out, and tends to crack fingernails. Fast action soap and a well-stocked first aid stand help if contact happens. The one time someone didn’t wear gloves, it took weeks for his hands to heal. Mask up if there’s a chance of dust—the powder becomes airborne when sliced or ground. Single use gloves always go straight to a chemical waste bin, not the general trash.
Even small spills get treated like a high-alert event. Absorbent pads soaked in sodium thiosulfate neutralize it fast. Companies provide bench sheets for just this reason—don’t learn the hard way burning a hole through plastic. For larger accidents, every facility needs dedicated spill kits and clear emergency plans. Training matters; don’t just rely on a binder sitting in a cabinet. Drills every year keep skills sharp. Emergency eyewash and shower—can’t skip the walk-throughs to make sure they're working.
Chemical waste holding tanks come into play right away. Don't pour benzoquinone down the drain—it damages water treatment bacteria and throws off the balance. EPA rules say it’s hazardous waste; expect fines if you ignore them. Sealed it up tight, label, store, and contact certified pickup. At the last lab I worked, the waste calendar sat above every bench, so everyone kept to a schedule. That kind of discipline saves trouble down the line.
Anyone who’s spent time in a college chemistry lab probably remembers the sharp, sweet smell of 1,4-benzoquinone. Yellow-orange crystals, easy to spot, often tucked away in brown bottles to keep them from degrading. Not just an academic curiosity, this compound finds a place in industries ranging from photography to pharmaceuticals. For anyone hoping to work with it—whether tinkering with redox reactions or building electrochemical sensors—one question comes up early: how does benzoquinone behave when mixed with different solvents?
A lot of folks expect small organic molecules to jump right into water, but benzoquinone is picky. Sitting in a beaker of cold water, these crystals stubbornly hold their shape. Only about 1.85 grams seep into a liter at room temperature. The oxygen atoms on the quinone ring pull some water molecules close, but the rest of the ring keeps things at arm’s length. So, if someone wants to work with it in water, especially at higher concentrations, they’ll run into a wall pretty quickly.
The real magic happens in organic solvents. Drop benzoquinone into ethanol, methanol, or acetone and it dissolves without a fuss. I remember the first time running a reaction in acetone—it took just seconds for everything to clear up. That’s the kind of solubility that opens doors. Researchers rely on this solubility to carry out oxidations, study electron transfer, or work up analytical procedures. In dichloromethane or ether, benzoquinone still plays along, only showing a little less enthusiasm. Lab manuals often recommend using these solvents for recrystallization or as carriers in thin-layer chromatography because they pull benzoquinone into solution quickly, and getting crystals back is usually just a matter of evaporating the liquid.
Higher solubility in organics means benzoquinone also travels more easily across cell membranes. That’s interesting for researchers exploring its cytotoxic properties but means extra care for anyone working at the bench. Skin contact or inhalation can cause irritation or even more serious toxicity. Wearing gloves and working under a fume hood turns into a basic requirement. Quick dissolution in organic solvents also pushes folks to think hard about spill control and waste handling, especially in shared spaces where cross-contamination could ruin sensitive experiments. Mixing strong solvents with oxidizers has sent more than one careless chemist to the safety shower.
Not everyone has access to pure organic solvents or wants to use them. In fields like green chemistry, solvent choice becomes almost as important as the chemistry itself. Water, with its good safety profile, loses appeal because benzoquinone won’t dissolve enough without a cosolvent. Researchers have begun leaning on mixed solvent systems, adding small bits of ethanol, or using surfactants to boost the compound’s solubility. Making use of these solutions, I’ve often found myself combining a little ethanol with water to coax benzoquinone into doing what I want without sacrificing too much in terms of safety or sustainability.
Anyone hoping to work efficiently with this molecule faces a choice. Staying safe in the lab and hitting the right concentrations often comes down to picking the right solvent. For redox studies, batteries, or dyes, ethanol or acetone nearly always come through. In greener settings, creative mixture design keeps experiments on track. My own experience says that knowing these solubility tricks isn’t just a box to check on a safety sheet—it’s a daily tool that keeps experiments humming and everyone healthy.
| Names | |
| Preferred IUPAC name | Cyclohexa-2,5-diene-1,4-dione |
| Pronunciation | /ˌbɛn.zoʊ.kwɪˈnoʊn/ |
| Identifiers | |
| CAS Number | 106-51-4 |
| Beilstein Reference | 3599809 |
| ChEBI | CHEBI:15801 |
| ChEMBL | CHEMBL1409 |
| ChemSpider | 505 |
| DrugBank | DB04170 |
| ECHA InfoCard | 01b891087901-40fc-4129-b0ce-829fa252a6c2 |
| EC Number | 1.6.5.6 |
| Gmelin Reference | 60872 |
| KEGG | C00196 |
| MeSH | D001567 |
| PubChem CID | 865 |
| RTECS number | DQ9625000 |
| UNII | WZK9C61OHV |
| UN number | 2587 |
| CompTox Dashboard (EPA) | DTXSID7020082 |
| Properties | |
| Chemical formula | C6H4O2 |
| Molar mass | 108.095 g/mol |
| Appearance | Yellow crystalline solid |
| Odor | pungent |
| Density | 1.32 g/cm³ |
| Solubility in water | 6 g/100 mL (20 °C) |
| log P | 1.01 |
| Vapor pressure | 5 mmHg (20 °C) |
| Acidity (pKa) | 1.64 |
| Basicity (pKb) | 7.04 |
| Magnetic susceptibility (χ) | -42.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.552 |
| Viscosity | 0.956 mPa·s (25 °C) |
| Dipole moment | 2.01 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 'Vapour: 299.0 J·mol⁻¹·K⁻¹' |
| Std enthalpy of formation (ΔfH⦵298) | +112.5 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | –1310 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D06AX08 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS02, GHS06 |
| Signal word | Danger |
| Hazard statements | H301, H311, H315, H319, H330, H335, H341, H373 |
| Precautionary statements | P210, P264, P280, P302+P352, P305+P351+P338, P310, P321, P332+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | '2-2-2-W' |
| Flash point | 79 °C (174 °F; 352 K) |
| Autoignition temperature | 250 °C |
| Explosive limits | 4.7–21% |
| Lethal dose or concentration | LD50 (oral, rat): 130 mg/kg |
| LD50 (median dose) | 130 mg/kg (rat, oral) |
| NIOSH | CN1400000 |
| REL (Recommended) | 0.15 |
| IDLH (Immediate danger) | 50 ppm |