Looking back at the late 19th century, organic chemists spent long nights puzzling over coal tar fractions. They isolated all kinds of aromatic compounds, including naphthalene, which paved the way for discovering a maze of quinones. Around this time, work on naphthoquinones ramped up as researchers uncovered their dyeing abilities and strange reactivity under mild conditions. By the early 20th century, scientists already started experimenting with naphthoquinone cores for vitamins and other intriguing biological activities. With the birth of synthetic chemistry, chemists figured out how to introduce specific double-bonded oxygens to naphthalene rings, bringing reliable access to 1,2-naphthoquinone. Years later, this work shaped synthesis routes for drugs, insecticides, and specialty chemicals. Today’s research still borrows tools and ideas first tested generations ago.
1,2-Naphthoquinone, sometimes called ortho-naphthoquinone, forms the backbone of many specialty ingredients in plant protection, dye intermediates, and analytical reagents. This molecule consists of two oxygen atoms double-bonded to a naphthalene ring at the 1 and 2 positions. Many labs order this compound for both routine syntheses and new experiments, relying on its punchy reactivity and ability to undergo numerous transformations. Labs often store it as a yellow crystalline solid, and producers supply it with certificates of analysis and purity controls reflecting years of standardization.
The yellow crystals of 1,2-naphthoquinone display a melting point near 115°C, with low solubility in water but better compatibility with acetone, diethyl ether, and hot ethanol. Its sharp odor stands out, and exposure to air sometimes darkens the powder. Chemists notice strong absorption in UV-visible spectra, often using this clue for purity checks. Its chemical backbone—two ketone groups on a naphthalene scaffold—unleashes a whole range of electron transfer reactions, especially important for organic redox studies. Its volatility remains moderate, so it rarely evaporates at room temperature, but heating triggers decompensation, releasing noxious fumes.
Suppliers grade 1,2-naphthoquinone by purity, water content, and residual solvents. Reagent grade usually means at least 98% purity, with GC, NMR, and HPLC used for tracking impurities and confirming structure. Drum or bottle labels must highlight the chemical name, CAS number (82-91-3), hazard pictograms, and GHS classification ("harmful if swallowed or inhaled"), as mandated by international standards. Standard shipping includes safety datasheets with details on storage and accidental release. Researchers check these details every time, especially before scaling up or performing public-facing work. Quality control batches list synthesis date and expiration, confirming identity by IR and MS.
Chemists typically oxidize 1,2-dihydroxynaphthalene to obtain 1,2-naphthoquinone. Air or chemical oxidants—such as silver oxide, ferric chloride, or even hydrogen peroxide—drive the transformation. Some old textbooks mention mild nitric acid for small-scale lab runs, though this route falls out of favor due to side reaction headaches and poor yields. Modern industry often prefers safer oxidants and controlled thermal processing to handle larger production with fewer impurities. Rotavapors, reflux columns, and glass reactors play key roles in both research and manufacturing operations. Crude products undergo vacuum drying, repeated filtration, and sometimes column chromatography to get bright yellow, high-purity crystals.
Few molecules offer the same versatility as 1,2-naphthoquinone’s dicarbonyl system. The two double-bonded oxygens attract nucleophiles—such as amines and thiols—to create all sorts of substitution reactions. Amines form imino derivatives, expanding the pool of medicinal candidates and antioxidant research. Metal-catalyzed hydrogenation converts the compound into naphthols for further coupling science, while halogenation opens up routes to more exotic quinoid intermediates. Diels-Alder reactions allow skilled hands to stitch new rings onto the naphthalene core, spawning building blocks for synthetic dyes and agrochemicals. Each transformation teaches chemists new tricks for managing electron flow in aromatic frameworks.
1,2-Naphthoquinone goes by several aliases: ortho-naphthoquinone, α-naphthoquinone, and o-naphthoquinone. International markets sometimes tag the product as NQ-12, or by catalog numbers tied to each chemical supplier. Laboratories double-check synonyms before ordering because mix-ups with 1,4-naphthoquinone (para isomer) can disrupt months of work.
1,2-Naphthoquinone can irritate skin, eyes, and lungs, so chemists don lab coats, nitrile gloves, and goggles before even opening a vial. The solid should never be handled in open air; fume hoods take priority, and labs keep spill kits at the ready. If inhaled, its dust might trigger coughing or more severe symptoms. GHS labeling rings especially true for this quinone, since improper disposal affects both human health and aquatic environments. Training for handling and disposal never ends; annual refreshers help prevent accidents and manage waste. Exhaust fans and cold storage cabinets limit volatility, and storage away from acids and bases prevents unintended reactions.
Pharmaceutical researchers probe 1,2-naphthoquinone for its antimalarial and anticancer properties, with several promising leads showing activity against parasites and tumor cells. In agriculture, scientists have mixed the compound into fungicidal or insecticidal formulations that fight crop disease without heavy metals or persistent toxins. Analytical chemists reach for the compound during redox titrations—it serves as a reference molecule to measure electron transfer. The dye industry values its deep yellow hue in creating pigments and color-fast compounds, while environmental labs use it to probe pollutant behavior or mimic certain metabolic reactions in soil and water. Even electronics work seizes on the compound's ability to shuttle electrons, inspiring research on organic conductors and solar cell ingredients.
Labs worldwide design new derivatives of 1,2-naphthoquinone, tuning its substituents for enhanced biological or electrical properties. There’s ongoing progress in turning this aromatic core into stronger enzyme inhibitors or targeted drug leads, often involving computational modeling and high-throughput screening. Chemical engineers try to improve yields and lower byproducts by swapping out classical oxidants for greener alternatives, such as oxygen with mild metal catalysts. Some projects stretch into materials science, where researchers blend the quinone with polymers to craft flexible batteries or organic semiconductors. Open-access journals showcase regular breakthroughs, but patent filings show fierce competition and huge investments from major chemical and pharmaceutical players.
Exposure studies track the fate of 1,2-naphthoquinone inside cells and whole organisms. Some findings tie chronic inhalation to respiratory inflammation, particularly for people working with aromatic hydrocarbons. Animal studies warn about liver and kidney stress at high doses, while fish and aquatic insects show vulnerability at much lower levels. Scientists dig into these effects with both cell cultures and animal models, tracing metabolic breakdown and tracking activated oxygen species. They publish protocols for spill response and decontamination, urging industry to stick to strict exposure limits and encourage strong monitoring programs. Biodegradation studies lead to better treatment tech in wastewater plants and help draft tighter regional regulations.
Momentum builds for greener production methods—catalysts that cut waste, lower energy, and spare toxic reagents. Biotech teams hope engineered bacteria will someday handle the quinone's synthesis straight from sugar feedstocks, avoiding fossil fuels altogether. Electronics research may soon deliver new flexible devices or batteries thanks to the compound’s redox properties, blurring the line between old-school chemistry and emerging tech. Drug discovery rides on increased understanding of 1,2-naphthoquinone’s toxicology, aiming for safe therapeutic leads with minimized side effects. Policy shifts and consumer pushback against harsh pesticides and pollutants guide more manufacturers to reexamine every molecule in their processes. As these changes ripple outward, 1,2-naphthoquinone keeps its unique spot in both classic chemistry sets and cutting-edge experiments pushing toward a safer, cleaner future.
Ask someone on the street about 1,2-naphthoquinone and you’ll likely get a blank stare. But this yellowish compound pops up in plenty of places that affect our everyday life, sometimes without us knowing. The science world knows it as a key intermediate in both research and industry. From personal experience working around chemical labs, I’ve seen its impact not just as a research tool, but as a building block for products that touch everything from health care to environmental safety.
Drug development depends heavily on reliable building blocks, and researchers often turn to 1,2-naphthoquinone while searching for treatments ranging from antimicrobial agents to cancer drugs. Its chemical structure lends itself to modifications, which in turn helps chemists tailor properties for better biological activity. For example, naphthoquinone derivatives inspired by this compound show promise against malaria and certain tumors. Scientists test changes at different points on the naphthoquinone ring, hoping to find molecules with strong healing abilities and fewer side effects.
Chemistry lab courses use 1,2-naphthoquinone a lot. In college, I remember it featured as a reagent for spotting amines or detecting certain metals in samples. It reacts in clear, predictable ways, allowing scientists to confirm whether a particular group or metal is present. Its sharp color changes make it especially useful in analytical methods, including thin-layer chromatography and colorimetric testing—a practical aspect of chemistry that moves findings from the page right into visual confirmation.
Color plays a big part in how we experience the world, and this compound adds to that palette. Its backbone forms the basis for several dyes, which show up in fabrics, plastics, and even inks. Textile chemists lean on naphthoquinone derivatives for both rich shades and longer-lasting colors. Over the years, these dyes have migrated beyond clothing, surfacing in color-sensitive coatings and imaging materials.
Environmental scientists watch 1,2-naphthoquinone closely. It occurs as a degradation product of naphthalene, a component of urban air pollution. Research groups track it in city air and rainwater, since it has links to oxidative stress in lung cells—a concern for public health workers. Tools designed years ago to monitor airborne pollutants now include techniques centered around pinpointing naphthoquinones. This gives communities a better picture of what they’re breathing in.
No discussion of this chemical would be responsible without acknowledging its downsides. High concentrations can lead to cell toxicity. Studies from public health groups warn about its contribution to oxidative stress and potential DNA damage. Laboratories handling this compound adopt strict safety procedures, including fume hoods and gloves. There’s always a push to find safer alternatives for industrial processes and to tighten air quality standards in cities where pollution levels are high.
1,2-Naphthoquinone doesn’t make headlines, but its influence stretches from hospital labs to city streets. By looking at how it’s used—and where caution is needed—we see the quiet ways chemistry shapes public health, industry, and scientific discovery. Keeping an eye on safety and exploring greener options helps make sure its benefits don’t come with unnecessary risks.
1,2-Naphthoquinone stands out with its deep yellow to orange crystalline appearance. Bring this compound into the lab and the first thing that pops is its sharp, bitter odor—not exactly the inviting scent you’d want lingering on your clothes. Scoop some powder and it’ll stain your hands with an unmistakable hue, underlining its chromatic punch. At around 190°C, it starts to melt, which means you don’t need wildly extreme conditions to turn it from solid to liquid. That threshold marks it suitable for several synthesis reactions, especially when you need substances that transform efficiently in moderate heat.
If you set out to dissolve it, don’t grab a glass of water. 1,2-Naphthoquinone hates water and resists mixing. Organic solvents tell a different story, though. Ether, chloroform, and benzene bring out its solubility, drawing the molecule into solution almost eagerly. This speaks volumes in the world of chemistry, as ease of solubility in non-polar solvents boosts usefulness in organic synthesis and research.
This molecule owns up to its quinone family roots. Two carbonyl groups anchored at the 1 and 2 positions give it both an edge in reactivity and in biological interest. Oxidation-reduction reactions come naturally. This stuff grabs electrons from other molecules and passes them along with a kind of efficiency you don’t see in less-oxidized aromatics. Quinones like this play a supporting role in numerous redox processes—think energy cycling in cells (as in ubiquinones and vitamin K derivatives).
Exposing 1,2-Naphthoquinone to a strong reducing agent like sodium borohydride leads to conversion into 1,2-dihydroxynaphthalene. Chemists count on this kind of versatility for making dyes, pharmaceuticals, and various polymer intermediates. There’s a time in nearly every chemistry student’s life where handling this molecule—or its close cousins—means learning what it feels like to genuinely control oxidation states rather than watching theory from the sidelines.
Don’t overlook the electrophilic properties of these carbonyl groups either. Nucleophiles, whether coming from water or alcohols, attack the electrophilic centers, allowing for ring-opening or further modification. This provides a window into synthesizing functionalized aromatic systems—a bread-and-butter principle for both material and medicinal chemists.
1,2-Naphthoquinone’s chemistry isn’t just academic. Research connecting it with air pollution and oxidative stress raises important health questions. Found among the degradation products of naphthalene in some urban environments, this compound has become a focus in toxicology research. It interacts strongly with cellular proteins and enzymes, contributing to toxicity through oxidative mechanisms. Evidence shows that exposure can damage DNA or interfere with critical cellular processes in lungs and other tissues. This isn’t just textbook chemistry—this is real world impact, and it elevates the need for tight regulations and vigilant monitoring.
Better monitoring of air and water for naphthoquinone keeps communities safer. Investing in filtration systems at the industrial source or retrofitting chemical plants with more effective scrubbers can prevent release. Education on safe handling in the lab also goes a long way. On the research front, developing less persistent analogs might offer similar utility but break down faster in the environment, lowering risk to human health. Leveraging these strategies brings both safety and progress to the table—a rare win-win in chemical management.
Working in lab environments for years teaches you that not every chemical commands the same respect. 1,2-Naphthoquinone, a yellow crystalline compound, demands attention. It pops up in dye manufacture and certain organic syntheses, but its tendency to irritate the skin, eyes, and respiratory system sets it apart from less reactive substances. The fumes sting your nose, and even a brief exposure can leave a mark. These risks aren’t just hype—case reports and material safety documentation lay out the facts. The compound’s toxicity may trigger headaches, nausea, or worse. No corner gets cut when it lands on the workbench.
Storage isn’t just about finding shelf space. 1,2-Naphthoquinone responds poorly to heat, light, or stray sources of ignition. A cool, dry storeroom with solid ventilation offers the bare minimum. Direct sunlight will speed up decomposition. I always put it in a tightly sealed glass or HDPE container, labeling the vessel clearly with hazard warnings. Letting vapors escape, even from a cracked lid, sours the air and puts others at risk. Creating a clear inventory, updated every time someone checks out a sample, isn’t just paperwork—it prevents nasty surprises.
Avoid placing it near oxidizers or acids. This precaution keeps tiny mistakes from turning into full-blown reactions. Some labs even dedicate separate cabinets for organics like naphthoquinone, fitted with built-in spill containment. In one previous workplace, we placed the containers inside secondary plastic bins—catching drips if someone got clumsy. Back home, the lesson holds for other household chemicals: separation limits disaster.
Every chemist who’s grabbed a bottle with cracked gloves learns fast. Standard disposable gloves offer little resistance—choose more robust nitrile or neoprene. Goggles with side protection and a fitted lab coat round out the basics. Few things shake your confidence more than a splatter in the eye or on bare skin. Handwashing stations situated close to the storage area give you an extra layer of security.
Measuring or transferring 1,2-Naphthoquinone belongs inside a chemical fume hood, never out in the open. The fume hood pulls vapors out and away from you. Regular air monitoring to check for stray organics in the workroom builds peace of mind for lab staff. Some colleagues new to the field try shortcuts—“just this once," they say—but the risks don’t shrink based on familiarity.
Mistakes happen. Quick and cool heads matter most. If someone splashes naphthoquinone on their skin, they should wash immediately with plenty of running water. Eyes get rinsed for at least fifteen minutes using an emergency eyewash. Nobody should shrug off dizziness or headaches. Medical teams take these symptoms seriously, and so should anyone exposed.
Small spills get covered with a mix of inert absorbent, like vermiculite, and then scooped into a hazardous waste bag. Cleaning up with paper towels or bare hands won’t cut it. For bigger spills, alert the building safety crew without delay. Good training and regular practice drills make all the difference—people remember steps better after real-world scenarios, not because they watched a video once.
Proper handling and storage practices come down to more than protocol—they represent respect for coworkers, the environment, and your own well-being. After years in research, the best labs I’ve seen rely as much on shared vigilance as on fancy equipment. Regular safety audits, accessible training, and a willingness to call out sloppy practices keep the worst from happening. Protecting yourself and others while working with 1,2-Naphthoquinone isn’t just a box to check; it’s one genuine way to show you care.
1,2-Naphthoquinone belongs to a class of organic compounds called quinones, often linked to industrial applications, including dyes, pigments, and even some aspects of chemical manufacturing. Coming across this compound usually happens in laboratory or factory settings. You don’t see it show up in everyday household products, but it can sneak its way into the air through some forms of pollution.
The main ways people might run into 1,2-Naphthoquinone are through breathing it in, skin contact, or possibly swallowing. Workers in industrial plants or labs see the highest risk. City dwellers can come into contact, since this compound forms as a byproduct in air pollution— especially from car exhaust and cigarette smoke.
Reports from places like the National Institute for Occupational Safety and Health (NIOSH) show that short-term inhalation can start to irritate the eyes, skin, and throat. People working with chemicals long enough don’t ignore burning, watering eyes, or an itchy nose—they know these are warning signs. Inhaled naphthoquinones can lead to coughing or wheezing. Studies also suggest possible asthmatic effects and decreased lung function with repeated exposure.
This compound brings bigger concerns for the oxidative stress it stirs up inside the body. Oxidative stress triggers inflammation and cell damage. Scientists at UC Davis and elsewhere have published research showing how 1,2-Naphthoquinone can disrupt cell function at low concentrations by generating reactive oxygen species. For someone with asthma or another lung condition, this kind of stress adds up quickly—sometimes causing aggravated flare-ups.
A lot of air pollution research circles back to the same problem: chronic exposure wearing people down over time. For 1,2-Naphthoquinone, that means links to higher rates of bronchitis, diminished lung growth in kids, and possibly even increased cancer risk with repeated, high-dose exposure. The International Agency for Research on Cancer hasn’t declared it a human carcinogen outright, but long-term effects seen in animal models leave little doubt about the need to limit chronic contact.
Anyone working with chemicals knows the drill: gloves, goggles, good ventilation. Skin or eye contact brings pain and irritation and should push anyone to wash up immediately and leave any contaminated clothing behind. People who get this compound in their eyes typically seek emergency rinsing for a full 15 minutes or more, then see a doctor.
Inhalation incidents usually need fresh air right away, and sometimes oxygen or further medical treatment. Swallowing is much less likely but, if it happens, getting medical help straight away beats any home remedy.
Reducing health risks starts with containment and substitution, wherever possible. Factories swapping to less hazardous alternatives see fewer workers getting sick. Well-maintained ventilation and filtering systems help stop vapors from building up. Wearing the right personal protective equipment actually makes a difference. Strong worker training lowers the odds of someone forgetting safe handling rules in the first place.
Beyond the factory floor, tighter air quality controls and vehicle emission standards go a long way. Urban planners can draw on public research that proves the health costs of letting pollution linger. Support for green technologies and cleaner fuels cuts back on all quinones, not just this one.
Access to up-to-date safety data, regular health checkups for workers, and real enforcement of existing chemical safety laws keep the worst cases from turning up in the first place. That’s the kind of groundwork that turns factory safety protocols into habits—and public health warnings into action people take seriously.
1,2-Naphthoquinone holds a spot in many chemical labs, thanks to its role in research and industrial synthesis. People use it to study oxidation reactions and to create dyes, batteries, and even certain pharmaceuticals. Shoppers looking for high-purity 1,2-Naphthoquinone need to move with care. That comes down to the potential hazards with quinones and strict laws controlling their purchase.
Finding a trusted chemical supplier makes all the difference. Sigma-Aldrich, Fisher Scientific, and TCI America serve researchers across the globe, keeping up certifications that meet standards like ISO or cGMP. Reputable sellers show detailed purity specs on their product pages, often with safety data sheets and batch test data. Most demand proof that the buyer belongs to a business or research institution, never shipping to a non-verified home address. This approach keeps buyers safe and follows legal rules.
When buying compounds for research, the degree of purity listed isn’t just a bragging point—it can make or break an experiment. For 1,2-Naphthoquinone, technical grade usually works for industrial processes, but research centers demand 98% purity or above. Impurities lead to data that doesn’t hold up and skew manufacturing results. Labs care about trace metals and water content, which trusted vendors include in their technical specs.
I’ve ordered specialty chemicals before, and a solid supplier always asks a few questions up front. Who is buying? For what project? Does the lab have a chemical hygiene officer? Many top vendors reject orders if these answers don’t check out, even if the customer waves cash. This screening builds trust and keeps supply chains away from shady projects. Places cutting corners usually skip these questions, putting users and institutions at risk.
People sometimes look for shortcuts, especially if they spot sellers on online marketplaces with rock-bottom prices. That route brings legal and personal trouble. Countries like the United States, the UK, and members of the EU list 1,2-Naphthoquinone under chemical management rules. Authorities have clear distribution chains for controlled chemicals. Listing a false address or forging credentials crosses the line from a shopping shortcut to criminal action. It’s not just paperwork—improper storage or handling can spark fires and trigger toxic exposure.
Before you even browse, check your country’s chemical purchasing laws. Many research-grade suppliers let you browse catalogs online, but a real purchase almost always needs institutional accounts, end-use declarations, and sometimes a sit-down with compliance officers. For companies outside major research hubs, regional chemical distributors step in as gatekeepers, often pairing supply with storage and safety advice. Joining professional networks, such as the American Chemical Society, can help connect buyers with vendors who offer not only the compound itself but also troubleshooting and support with regulatory filings.
Investing in staff training helps smaller organizations avoid common regulatory pitfalls. Teams that understand chemical hazard categories and proper paperwork move faster and face fewer rejected orders. I know labs that keep a cutter file listing reliable suppliers and their paperwork quirks—this habit pays off, saving days of confusion. For organizations always running into dead ends, teaming up with a university or research consortium can open doors closed to solo operators.
The bottom line: stick with trusted, transparent sources; make sure your paperwork is up to snuff; and avoid shortcuts. 1,2-Naphthoquinone isn’t a do-it-yourself mail-order kind of chemical. Safety, legality, and science all depend on doing things the right way.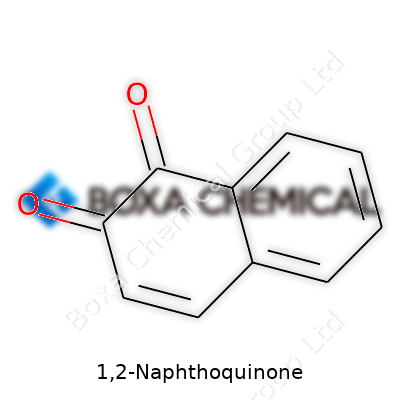
| Names | |
| Preferred IUPAC name | naphthalene-1,2-dione |
| Pronunciation | /ˌwʌnˌtuːˌnæfθəʊˈkwɪnoʊn/ |
| Identifiers | |
| CAS Number | 524-42-5 |
| Beilstein Reference | 1208739 |
| ChEBI | CHEBI:35362 |
| ChEMBL | CHEMBL156388 |
| ChemSpider | 6924 |
| DrugBank | DB03815 |
| ECHA InfoCard | 100.011.025 |
| EC Number | 207-432-0 |
| Gmelin Reference | 82211 |
| KEGG | C01754 |
| MeSH | D017894 |
| PubChem CID | 7064 |
| RTECS number | QJ9625000 |
| UNII | NMR0B8G5MR |
| UN number | UN2662 |
| CompTox Dashboard (EPA) | urn:lsid:epa.gov:compToxDashboard:9829 |
| Properties | |
| Chemical formula | C10H6O2 |
| Molar mass | 158.15 g/mol |
| Appearance | yellow crystalline solid |
| Odor | Odorless |
| Density | 1.42 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 1.67 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 4.5 |
| Basicity (pKb) | 7.74 |
| Magnetic susceptibility (χ) | -47.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.700 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.14 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 110.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 40.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2614 kJ/mol |
| Pharmacology | |
| ATC code | D05AC04 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P264, P280, P301+P312, P302+P352, P305+P351+P338, P330, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | '2-2-0' |
| Flash point | 113 °C |
| Autoignition temperature | 540 °C |
| Lethal dose or concentration | LD50 (oral, rat): 200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 200 mg/kg (rat, oral) |
| NIOSH | NA4850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.02 mg/m3 |
| IDLH (Immediate danger) | Not established |